Abstract
Introduction
Risankizumab is a humanized monoclonal antibody that selectively targets interleukin-23. It is approved for treatment of moderate-to-severe plaque psoriasis. We conducted a 52-week monocentric retrospective study to evaluate the effectiveness and safety of risankizumab in a real-life setting.
Methods
Our study included 131 adults with moderate-to-severe plaque psoriasis all treated with risankizumab for at least 52 weeks. Patient characteristics and PASI (Psoriasis Area and Severity Index) at each visit were recorded. The percentages of patients achieving 75%/90%/100% (PASI 75/90/100) improvement in PASI with respect to baseline were registered.
Results
At week 52, 93.9%, 78.6%, and 61.1% of patients achieved PASI 75/90/100, respectively. An absolute PASI ≤ 2 was reached by 90.8% at week 52. The higher body mass index and the presence of cardio-metabolic comorbidities did not interfere with the odds of reaching PASI 75/90/100 at each time-point. At week 52, comparable percentages of patients achieved PASI 100, regardless of the involvement of difficult-to-treat-areas. No significant safety findings were recorded and none of the patients had to interrupt the treatment because of adverse events.
Conclusions
Our findings confirmed that risankizumab is a safe and effective therapeutic option for the treatment of a wide “real-life” cohort of patients with psoriasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The efficacy and safety of risankizumab have been assessed in multiple clinical trials and real-life studies, but not enough data are available on larger cohorts of patients with a longer follow-up. |
We conducted a real-life study on 131 patients, all treated with risankizumab for at least 52 weeks, with 26 of them completing 2 years of therapy to further evaluate the effectiveness and safety of this drug. |
In our experience, risankizumab proved to be equally effective in different subgroups of patients regardless of BMI status and presence of cardio-metabolic comorbidities. |
Risankizumab appears to be a safe therapeutic option in a wide cohort of patients, including those with a previous history of malignancies and serological evidence of viral hepatitis B and C. |
Longer studies with larger cohorts of patients are needed to further assess this subject. |
Introduction
Psoriasis is a common inflammatory disease that affects from 2 to 4% of the general population worldwide [1]. It primarily affects the skin, but it is now well established as a multisystemic disease that can also affect the immune system, the joints, and the cardiovascular system. On the skin, psoriasis usually presents itself as psoriasis vulgaris with erythematous scaly plaques and patches which most commonly involve the elbows, the knees, the scalp, and the lumbosacral region [2, 3]. However, plaque psoriasis can occur anywhere on the skin, involving also sensitive areas, such as the genitals, face, palms/soles, and nails. Psoriasis can greatly impair the well-being and the quality of life of patients. Most of the cases present as mild or moderate conditions, with the involvement of less than 10% of body surface area (BSA). Moderate-to-severe cases, with the involvement of sensitive areas or more than 10% of BSA, affect 20% of the patients [4, 5].
Treatments for mild-to-moderate plaque psoriasis include topical corticosteroids and vitamin D3 derivates. When managing moderate-to-severe cases, the first-line therapies are represented by conventional immunosuppressive drugs, such as cyclosporine, methotrexate, and acitretin [6]. When a patient has a contraindication or an incomplete response to these drugs, biologics are the treatment of choice [7, 8]. The advances in the knowledge of the pathogenesis of psoriasis have shifted the focus from the keratinocytes to the T-helper lymphocytes, who are the main players in the inflammatory cascade of plaque psoriasis. In particular, an important role is played by two interleukins (ILs): IL-17 and IL-23 [9]. During the last 4 years, three selective antagonists of the p19 subunit of IL-23 have been developed. Risankizumab is a humanized IgG monoclonal antibody that inhibits selectively the IL-23. Risankizumab has shown efficacy and safety in five phase 3 clinical trials (UltIMMa-1, UltIMMa-2, IMMerge, IMMhance, and IMMvent) [10,11,12,13]. A few real-life experiences have also been published, confirming the data from the clinical trials [14,15,16].
The aim of our monocentric retrospective study was to assess the effectiveness and safety of risankizumab in 131 patients treated for at least 52 weeks with this drug, with a subset of 26 patients completing 104 weeks of therapy.
Methods
We conducted this monocentric retrospective study analyzing the electronic medical records of our Dermatology Unit at IRCCS Humanitas Research Hospital in Rozzano, Milan, Italy. We enrolled 131 patients who completed at least 52 weeks of treatment with risankizumab. All 131 patients received two subcutaneous injections of 75 mg risankizumab at weeks 0 and 4 and every 12 weeks after that, according to the summary of product characteristics [17]. Patients presented at our institute for dermatologic visits at baseline and at weeks 16, 28, 40, and 52. At week 4, all patients self-injected the drug at home after receiving training in subcutaneous injection techniques. Patients' demographics, comorbidities, previous systemic treatments, and Psoriasis Area and Severity Index (PASI) at each dermatological examination were retrieved from electronic medical records. Body mass index (BMI) class and cardio-metabolic comorbidities were also recorded. The involvement of difficult-to-treat areas was also recorded, including palms/soles, nails, face/scalp, and genitals.
The effectiveness of risankizumab was evaluated by assessing the percentages of patients who achieved 75, 90, and 100% (PASI 75, PASI 90 and PASI 100) improvement in PASI with respect to baseline PASI. Absolute PASI ≤ 2 was also selected as an efficacy endpoint, according to an Italian adaptation of EuroGuiDerm guidelines [8]. Due to the study’s retrospective nature, missing data could not be retrieved. For patients who did not attend the scheduled dermatological visits and performed the injection at home or missed the injection, the PASI of the last observation carried forward (LOCF) was considered.
The eventual occurrence of adverse events (AEs) was recorded at each dermatologic visit. All patients underwent routine blood chemistry test and screening for HIV, viral hepatitis, and TB Gold Quantiferon, before starting the treatment, according to Italian Guidelines.
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received risankizumab as in good clinical practice, in accordance with Italian guidelines. All included patients provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Statistical analysis was guided by the intention-to-treat principle with the full analysis set being 131 patients treated with risankizumab. Stata/SE 17.0 software was used for analysis, and Microsoft Excel was used to generate tables.
Continuous parameters were reported using frequency, mean and Standard Deviation (SD) values. Discrete parameters were reported as count and percentage. The percentage of patients achieving an absolute PASI ≤ 2 and PASI 75, PASI 90, PASI 100 responses with risankizumab treatment was examined in relation to various parameters: biologic-naïve versus biologic-experienced patients, BMI class, involvement of difficult areas, the presence of PsA (psoriatic arthritis), and cardio-metabolic comorbidities. The categorical variables were analyzed using the chi-square test, while the continuous variables were tested Student’s t test and Fisher's exact test where needed. Non-normal distributions were tested using the Kruskal–Wallis test. Statistical significance was defined as a probability value of less than 0.05.
Results
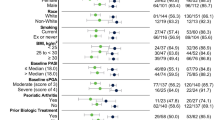
A total of 131 patients were enrolled in this study. All of them completed at least 52 weeks of treatment, with 26 of them being treated for 2 years with risankizumab. Ninety-two (70%) were males and 39 (30%) were females. Mean age was 51 years (15.64) and mean BMI was 28.77 kg/m2 (6.16) with 38.6% of patients being obese (BMI ≥ 30). Eighty-six patients (65.6%) had at least one comorbidity: 69 (52.7%) were affected by one or more cardio-metabolic comorbidities (including cardiovascular diseases, obesity, arterial hypertension, type two diabetes mellitus, and hyperlipidemia), 18 (13.7%) had psoriatic arthritis and nine (6.9%) had serological evidence of viral hepatitis (five patients with HBV and one patient with HCV) or a positive TB Quantiferon test.
A total of 69 patients (60.3%) were naïve to biologic therapies, and 62 (39.7%) had failed at least one biologic drug (bio-experienced). All of these 62 patients were switched to risankizumab following primary or secondary inefficacy of previous biologic drugs or because of treatment-related adverse events. Anti-TNFα drugs were previously prescribed to 22 patients (16.8%), ustekinumab to 19 patients (14.5%), while anti-IL17 were previously administered to 38 patients (29%) and other anti-Il-23 drugs (guselkumab, tildrakizumab) to eight patients (6.1%). Demographic characteristics of our population at baseline are summarized in Table 1.
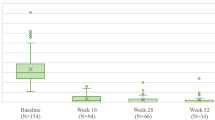
The mean absolute PASI scores at baseline and weeks 16, 28, 40, 52, and 104 are summarized in Fig. 1. Over the course of the study, the absolute PASI score decreased from a mean (SD) of 13.52 (8.14) at baseline to 1.75 (2.56) at week 16, 1.15 (1.85) at week 28, 0.79 (1.30) at week 40 and 0.62 (1.08) at week 52 (Fig. 1). Regarding the 26 patients who completed 2 years of treatment, their mean PASI (mPASI) at week 104 was 0.40 (0.72).
At week 16, 77.34, 55.73, and 36.64% of our 131 patients achieved PASI 75, PASI 90 and PASI 100, respectively, while an absolute PASI ≤ 2 was achieved by 75.57%. Those percentages were higher at week 28 when PASI 75 was reached by 82.44% of the patients, PASI 90 by 65.65%, PASI 100 by 47.33% and PASI ≤ 2 by 83.21%. At week 40, we observed PASI 75/90/100/ ≤ 2 responses in 88.55, 74.81, 54.20, and 87.79% of patients, respectively. At week 52, the percentages of PASI 75, PASI 90, PASI 100 and PASI ≤ 2 responses were 93.89, 78.63, 61.10, and 90.84%, respectively. At week 104, out of 26 patients, PASI 75 was reached by 96.15%, PASI 90 by 80.77%, PASI 100 by 69.23% and PASI ≤ 2 by 96.15%. Data regarding PASI 75, PASI 90, PASI 100, and PASI ≤ 2 of our cohort of patients are summarized in Fig. 1.
We also analyzed the therapeutic responses based on several parameters (Tables 2 and 3). Patients with a BMI < 25 had a baseline mPASI of 12.44, which was comparable with the mean PASI of overweight patients (14.33) and of obese patients (14.71), with a p value = 0.092 (Fig. 2). Regarding mPASI, in the group of patients with a BMI lower than 25, at week 16 the score was 1.06, at week 28 it was 0.73, at week 40 it was 0.52 and at week 52 the score was 0.48. In the cohort of overweight patients, mPASI at weeks 16, 28, 40, and 52 was 1.47, 1.16, 0.71, and 0.51, respectively. Finally, in the group of obese patients, the mPASI at week 16 was 2.61, at week 28 it was 1.80, at week 40 it was 1.33 and at week 52 the score was 1.03. No significant differences were observed throughout the study in the decreasing of mPASI regarding the BMI subpopulations. At each clinical examination, PASI 75, PASI 90 and PASI 100 responses did not show significant differences among the three subpopulations (Table 2). An absolute PASI ≤ 2 was reached by a significantly lower cohort of obese patients compared with overweight and normal weight patients at week 40 (75 vs. 92.3 and 96%, respectively, with a p = 0.041) and at week 52 (78.1 vs. 96.2 and 96%, respectively, with a p = 0.038).
In our study, psoriatic arthritis did not interfere with the clinical response to risankizumab, as comparable responses were observed at all visits regarding all efficacy endpoints (Fig. 3, Table 3). The mPASI scores were comparable between the two groups at baseline and at the subsequent visits, without any significant difference.
Regarding the previous exposition to biologic therapies (Fig. 4, Table 3), bio-naïve patients had a higher mPASI at baseline compared with bio-experienced patients (15.9 vs. 9.9, p < 0.001). No significant differences were observed throughout the study in the decreasing of mPASI regarding the two different subpopulations. At week 16, we observed a PASI 75 of 84.8% in the bio-naïve group compared with a PASI 75 of 63.5% in the group of bio-experienced (p = 0.005). At the same time point, PASI 90 was achieved by 68.4% of bio-naïve patients, compared with the 36.5% of bio-experienced (p < 0.001); PASI 100 was reached by the 46.8% of bio-naïve versus the 21.2% of patients previously treated with biologics (p = 0.003). Absolute PASI ≤ 2 was achieved by 83.5% of bio-naïve patients compared with 63.5% of bio-experienced population (p = 0.009). At week 28, significant differences between the group of bio-naïve and bio-experienced were observed regarding PASI 90 (77.2 vs.48.1%, p = 0.001) and PASI 100 (54.4 vs. 36.5%, p = 0.045). At week 40, a significantly higher percentage of bio-naïve subjects achieving PASI 75 (94.9 vs. 78.8%, p = 0.005) and PASI 90 (84.8 vs. 59.6%, p = 0.001) compared with bio-experienced patients was observed. At week 52, significant differences between the group of bio-naïve and bio-experienced were observed regarding PASI 75 (98.7 vs. 86.5%, p = 0.004) and PASI 90 (87.3 vs. 65.4%, p = 0.003).
In our study, the presence of cardio-metabolic comorbidities did not interfere with the clinical response to risankizumab, as comparable responses were observed at all visits regarding all efficacy endpoints (Fig. 5, Table 3). The only exception was represented by PASI ≤ 2 at week 16, which was achieved by more patients without those comorbidities (82.6 vs. 67.7%, p = 0.048). At the following visits, no differences were detected. mPASI scores were comparable between the two groups at baseline and at the subsequent visits, without any significant difference.
At baseline, 106 patients had psoriasis in difficult-to-treat areas, with 44 of them having cutaneous manifestations only on those sites (with a baseline PASI lower than 10). Those 44 patients, as expected, experienced lower PASI 75/90/100 responses at each time-point, as their mPASI at baseline was significantly lower (5.7) compared with both patients without involvement of difficult to-treat areas (15.92) and patients with high PASI scores and psoriasis at least in one difficult area (12.96) with a p < 0.001. At week 16, PASI75/90/100 scores were achieved by 80, 56, and 40% of patients without involvement in difficult areas, versus 88.7, 72.6, and 46.8% of patients with PASI > 10 and difficult areas involved. At this time point, the responses were significantly lower in patients with difficult-to-treat areas (56.8, 31.8, and 20.5%); p = 0.001, p < 0.001, p = 0.20, respectively. Regarding PASI ≤ 2, no significant differences were noticed among these groups during the whole study period. Despite better responses in PASI 75/90 in patients who did not present psoriasis in difficult areas at all the time points observed, regarding PASI 100 from week 28 onwards we did not any significant differences among the three groups (Fig. 6, Table 2).
Regarding the safety of risankizumab, none of our 131 patients had to discontinue the treatment because of adverse events (Table 4). Two patients reported headaches, one patient experienced erythema at the injection site, one woman reported rhinopharyngitis, and another patient had an episode of diarrhea. Despite our study being conducted in a period characterized by the spreading COVID-19 pandemic, no COVID-19-related hospitalizations, or deaths were reported during the whole study period. Regarding the three patients with a positive TB Quantiferon test, no signs of reactivation were detected at the annual thorax radiography and pneumologic visits. Although six patients had serological evidence of chronic viral hepatitis (five patients with HBV and one patient with HCV), follow-up laboratory tests and periodic hepatological visits showed no signs of viral reactivation during the treatment with risankizumab.
Discussion
Our study confirms the effectiveness of risankizumab in daily clinical practice in patients with moderate-to-severe plaque psoriasis, with the largest cohort of patients reaching at least 52 weeks of treatment, to date. Compared with the UltIMMa-1 and UltIMMa-2 clinical trials, the characteristics of our patients were similar, aside from having a lower baseline PASI score, which is due to the stricter inclusion criteria of phase 3 clinical trials [10]. Our population, however, was very similar to those analyzed by some real-life studies, including a Czech study from Gkalpakiotis et al. (with 154 patients at baseline) and a recent Italian study by Mastorino et al. (with 166 patients at baseline) [14, 18].
At week 16, we observed lower rates of PASI90 and PASI100 compared with phase 3 clinical trials. In particular, PASI 90 was achieved by 55.7% of patients at week 16, compared with UltIMMa-1 (75.3%), UltIMMa-2 (74.8%), IMMerge (73.8%). However, at week 52, compared to the UltIMMa-1 and 2 studies, the percentages of patients achieving PASI 100 and 90 in our population were comparable or slightly higher (56.3 and 50.7 vs. 61.1% for PASI 100; 81.9 and 80.6 vs. 78.6% for PASI 90) [10, 11]. Our data show a slower response compared with registration studies, with comparable or better rates of PASI 90 and PASI 100 at week 52. These findings are very similar to those observed by other real-life studies, with comparable populations. This could be partially due to the lower mPASI at baseline of real-life patients (13.52 vs. 20.6 and 20.5 in UltIMMA1 and 2). In our study, the absolute PASI score decreased from a mean (SD) of 13.52 (8.14) at baseline to 0.62 (1.08) at week 52, demonstrating an efficacy maintained over time, with an mPASI of 0.40 (0.72) at week 104 for the 26 patients who completed 2 years of therapy.
In our study, we did not observe any significant difference in response to risankizumab therapy regarding the BMI of the patients, with comparable PASI 75/90/100 responses throughout the study among the three subsets of patients (obese, overweight and normal weight patients). Our data are consistent with the results reported by Gkalpakiotis et al. who found no significant differences in patients with higher BMI compared with normal weight patients [14]. Conversely, both Mastorino et al. and Hansel et al. observed a lower response in the overweight or obese population [16, 18]. It is worth noticing, however, that these real-life studies used a different stratification for BMI, analyzing only obese versus non-obese populations. It is well known that a high BMI is a negative predictor of response to some biological treatment as anti-TNFα, ustekinumab, and secukinumab, while other anti-IL-23 and anti-IL-17 appeared to not be influenced by body weight [19, 20]. Our data support the efficacy of risankizumab also in obese and overweight patients, confirming the results of subgroups analyses from the IMMerge study [21].
Similarly, in our study, no significant differences regarding the effectiveness of risankizumab were observed between patients with and without a diagnosis of PsA. Our data are in contrast with those observed by Mastorino et al. who found a significantly lower response rate at several time points in patients with PsA. To date, risankizumab has not been approved for the treatment of PsA in Europe, yet [18].
Comparing our data between the two cohorts of bio-naïve and bio-experienced patients, no significant differences emerged regarding the mPASI decrement throughout the study observation, despite bio-naïve patients having a higher mPASI compared with bio-experienced patients at baseline. We observed significant differences in the achievement of several efficacy endpoints (PASI75/90/100) at weeks 16, 28, 40, and 52. This could be explained by the high percentage of previous multi-failures and failures with anti-IL17 drugs among our bio-experienced cohort. However, at week 52, an absolute PASI ≤ 2 was achieved by 92.4% of bio-naïve patients compared with 88.5% of bio-experienced population (p = 0.444), showing no significant differences between the two populations. Bio-experienced patients started therapy with risankizumab directly switching from another biologic drug, explaining why the baseline PASI was lower compared with the bio-naïve cohort. The achievement of PASI ≤ 2 in a higher percentage of bio-experienced patients can be considered a relevant result in terms of patients' satisfaction and improvement of the quality of life since we have to consider patients who failed one or more biologic therapies refractory to an optimal response, due to the presence of difficult areas affected, comorbidities, high BMI or the assumption of other drugs [15, 22].
Regarding the impact of cardiovascular diseases and/or metabolic comorbidities on the response to risankizumab of patients with moderate-to-severe plaque psoriasis, interestingly, our data showed no significantly different responses for all efficacy endpoints (the only exception being a higher percentage of patients reaching an absolute PASI ≤ 2 at week 16). Our data confirm the analyses of a multicentric study conducted by Borroni et al. [15]. These findings support the effectiveness of anti-IL-23 drugs in patients with cardio-metabolic diseases, as a pooled analysis from the extension open-label studies on tildrakizumab recently described no significant differences in patients with and without metabolic syndrome [23].
Treatment of difficult-to-treat areas in psoriasis is still challenging [4]. In this study, we analyzed three groups of psoriatic patients, stratified on the baseline PASI and the presence or not of psoriasis in difficult areas: patients with PASI > 10 without any involvement of difficult areas, patients with PASI > 10 and at least one difficult area affected and patients with exclusive involvement of difficult-to-treat areas. At week 16, we observed a better response regarding percentage reduction of PASI in patients with PASI > 10 at baseline regardless the involvement of difficult areas. However, when focusing on absolute PASI, at any time point we did not observe any significant differences between the three groups of patients. Notably, all the population achieved comparable PASI 100 responses from week 16 to week 52, showing the effectiveness of risankizumab also on the exclusive involvement of difficult-to-treat areas, even in patients with a baseline PASI < 10.
In our study, risankizumab showed no significant safety findings, and up to week 52, no relevant side effects were reported among all patients receiving risankizumab (Table 3), in accordance with both the clinical trials and other real-life studies [10,11,12,13,14,15,16]. Regarding the 26 patients who completed 2 years of treatment, significant adverse events were described. Our data are consistent with other studies and reviews regarding the safety profiles of anti-IL-23 drugs [24,25,26]. Moreover, despite the current data being collected during the first outbreak of the COVID-19 pandemic, in an area strongly affected by SARS-CoV-2, none of the patients experienced severe forms of COVID-19. None of the six patients with serological evidence of viral hepatitis experienced viral reactivation during risankizumab, also confirming other published data regarding the safety of anti-IL-23 drugs in this population [27].
Our study has some limitations, the first one being its retrospective design, which does not allow the retrieval of missing data. Other significant limitations are the lack of a randomized controlled setting and the heterogeneity of clinical assessment among different clinicians.
Conclusions
Our study confirmed the effectiveness of risankizumab throughout a 52-week period, with higher therapeutic responses at week 28, 40, and 52 compared to week 16. Our data demonstrate the high effectiveness of risankizumab in daily clinical practice in a large cohort of patients with moderate-to-severe plaque psoriasis, with the largest cohort of patients completing a full year of treatment.
Compared with phase 3 RCTs, our study found slightly lower PASI 75/90/100 responses at week 16 but comparable or even higher responses at week 52 supporting the role of risankizumab as an effective therapeutic option in real-world conditions. Moreover, we observed better responses at all time points compared with other real-life experiences. Percentual PASI reduction was higher in bio-naïve patients compared with bio-experienced patients, with similar proportions of subjects achieving an absolute PASI of 2 or lower. The effectiveness of risankizumab was comparable among patients with and without cardio-metabolic comorbidities and with or without PsA, confirming the results of other real-life studies on anti-IL-23 drugs. Risankizumab proved also to be effective also in patients with the involvement of difficult-to-treat areas, including those with the exclusive involvement of palms/soles, scalp, or genitals.
Risankizumab was well tolerated, with no significant safety findings reported throughout the study up to week 104. Larger and longer prospective studies and retrospective analyses of patient registries are needed to evaluate the safety and effectiveness of risankizumab further in a real-life setting.
References
Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–94. https://doi.org/10.1016/S0140-6736(14)61909-7 (Epub 2015 May 27 PMID: 26025581).
Narcisi A, Valenti M, Cortese A, et al. Anti-IL17 and anti-IL23 biologic drugs for scalp psoriasis: A single-center retrospective comparative study. Dermatol Ther. 2022;35(2): e15228. https://doi.org/10.1111/dth.15228.
Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–60. https://doi.org/10.1001/jama.2020.4006.
Pavia G, Gargiulo L, Cortese A, et al. Apremilast for the treatment of palmo-plantar non-pustular psoriasis: a real-life single-center retrospective study. Dermatol Ther. 2022;35(2): e15253. https://doi.org/10.1111/dth.15253.
Kivelevitch D, Frieder J, Watson I, Paek SY, Menter MA. Pharmacotherapeutic approaches for treating psoriasis in difficult-to-treat areas. Expert Opin Pharmacother. 2018;19(6):561–75. https://doi.org/10.1080/14656566.2018.1448788.
Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12(12):CD011535. Published 2017 Dec 22. https://doi.org/10.1002/14651858.CD011535.pub2
Nast A, Smith C, Spuls PI, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris—Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–98. https://doi.org/10.1111/jdv.16915.
Gisondi P, Fargnoli MC, Amerio P, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol. 2022;157(Suppl. 1 to No. 1):1–78. doi:https://doi.org/10.23736/S2784-8671.21.07132-2.
Valenti M, Narcisi A, Pavia G, Gargiulo L, Costanzo A. What Can IBD Specialists Learn from IL-23 Trials in Dermatology?. J Crohns Colitis. 2022;16(Supplement_2):ii20-ii29. https://doi.org/10.1093/ecco-jcc/jjac023.
Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–61. https://doi.org/10.1016/S0140-6736(18)31713-6.
Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–59. doi:https://doi.org/10.1111/bjd.19341.
Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–58. https://doi.org/10.1001/jamadermatol.2020.0723.
Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–86. https://doi.org/10.1016/S0140-6736(19)30952-3.
Gkalpakiotis S, Cetkovska P, Arenberger P, et al. Risankizumab for the treatment of moderate-to-severe psoriasis: real-life multicenter experience from the Czech Republic. Dermatol Ther (Heidelb). 2021;11(4):1345–55. https://doi.org/10.1007/s13555-021-00556-2.
Borroni RG, Malagoli P, Gargiulo L, et al. Real-life effectiveness and safety of risankizumab in moderate-to-severe plaque psoriasis: a 40-week multicentric retrospective study. Acta Derm Venereol. 2021;101(11):adv00605. https://doi.org/10.2340/actadv.v101.283.
Hansel K, Zangrilli A, Bianchi L, et al. A 52-week update of a multicentre real-life experience on effectiveness and safety of risankizumab in psoriasis. J Eur Acad Dermatol Venereol. 2022;36(2):e111–3. https://doi.org/10.1111/jdv.17656.
European Medicines Agency. Skyrizi (risankizumab): summary of product characteristics. 2019 [cited 2022 June 03]. http://www.ema.europa.eu/en/medicines/human/EPAR/skyrizi.
Mastorino L, Susca S, Megna M, et al. Risankizumab shows high efficacy and maintenance in improvement of response until week 52. Dermatol Ther. 2022;35(5): e15378. https://doi.org/10.1111/dth.15378.
Malagoli P, Dapavo P, Pavia G, et al. Real life long-term efficacy and safety of ixekizumab in moderate-to-severe psoriasis: a 192 weeks multicentric retrospective study - IL PSO (ITALIAN LANDSCAPE PSORIASIS) [published online ahead of print, 2022 May 31]. Dermatol Ther. 2022;e15608. https://doi.org/10.1111/dth.15608
Facheris P, Valenti M, Pavia G, et al. Brodalumab: a new way to inhibit IL-17 in psoriasis. Dermatol Ther. 2020;33(3): e13403. https://doi.org/10.1111/dth.13403.
Crowley JJ, Langley RG, Gordon KB, et al. Efficacy of risankizumab versus secukinumab in patients with moderate-to-severe psoriasis: subgroup analysis from the immerge study. Dermatol Ther (Heidelb). 2022;12(2):561–75. https://doi.org/10.1007/s13555-021-00679-6.
Bonifati C, Morrone A, Cristaudo A, Graceffa D. Effectiveness of anti-interleukin 23 biologic drugs in psoriasis patients who failed anti-interleukin 17 regimens. A real-life experience Dermatol Ther. 2021;34(1): e14584. https://doi.org/10.1111/dth.14584.
Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84(2):398–407. https://doi.org/10.1016/j.jaad.2020.09.047.
Singh S, Singh S, Thangaswamy A, Thangaraju P, Varthya SB. Efficacy and safety of risankizumab in moderate to severe psoriasis: a systematic review and meta-analysis. Dermatol Ther. 2021;34(1): e14487. https://doi.org/10.1111/dth.14487.
Valenti M, Pavia G, Gargiulo L, et al. Biologic therapies for plaque type psoriasis in patients with previous malignant cancer: long-term safety in a single- center real-life population [published online ahead of print, 2021 Feb 15]. J Dermatolog Treat. 2021;1–5. doi:https://doi.org/10.1080/09546634.2021.1886231
Dapavo P, Siliquini N, Mastorino L, et al. Efficacy, safety, and drug survival of IL-23, IL-17, and TNF-alpha inhibitors for psoriasis treatment: a retrospective study [published online ahead of print, 2021 Aug 13]. J Dermatolog Treat. 2021;1–6.
Gargiulo L, Pavia G, Valenti M, et al. Safety of biologic therapies in patients with moderate-to-severe plaque psoriasis and concomitant viral hepatitis: a monocentric retrospective study. Dermatol Ther (Heidelb). 2022;12(5):1263–70. https://doi.org/10.1007/s13555-022-00726-w.
Acknowledgements
We thank the participants of the study.
Funding
This work and the Rapid Service Fee were supported from grants from “Fondazione Roma”, Italian Ministry of Health (Rome, Italy), ‘Ricerca Finalizzata’ project number CO-2013–02356463.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Luigi Gargiulo, Luciano Ibba Giulia Pavia, Carlo Alberto Vignoli, Francesco Piscazzi, Mario Valenti, Federica Sanna, Chiara Perugini, Jessica Avagliano, Antonio Costanzo and Alessandra Narcisi. The first draft of the manuscript was written by Luigi Gargiulo, Luciano Ibba and Giulia Pavia, and all authors commented on previous versions of the manuscript. Funding acquisition: Antonio Costanzo Resources: Antonio Costanzo. Supervision: Alessandra Narcisi. All authors read and approved the final manuscript.
Disclosures
Antonio Costanzo has been a consultant and/or speaker for AbbVie, Almirall, Amgen, Janssen, Leo Pharma, Eli Lilly, Galderma, Boehringer, Novartis, Pfizer, Sandoz, and UCB. Alessandra Narcisi has been a consultant and/or speaker for AbbVie, Almirall, Amgen, Janssen, Leo Pharma, Eli Lilly, Boehringer, Novartis, Pfizer and UCB. Mario Valenti has been a consultant and/or speaker for Eli Lilly, Leo Pharma, Boehringer and Sanofi. Luigi Gargiulo, Luciano Ibba, Giulia Pavia, Carlo Alberto Vignoli, Francesco Piscazzi, Federica Sanna, Chiara Perugini, and Jessica Avagliano have nothing to disclose.
Compliance with Ethics Guidelines
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received risankizumab as in good clinical practice, in accordance with Italian guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gargiulo, L., Ibba, L., Pavia, G. et al. Real-Life Effectiveness and Safety of Risankizumab in 131 Patients Affected by Moderate-to-Severe Plaque Psoriasis: A 52-Week Retrospective Study. Dermatol Ther (Heidelb) 12, 2309–2324 (2022). https://doi.org/10.1007/s13555-022-00795-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00795-x