Abstract
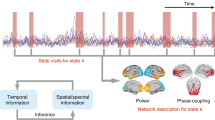
It has been reported that cross-frequency interactions may play an important role in local processing within thalamus and neocortex, as well as information transfer between subcortical and cortico-cortical brain regions. Strong commonalities in rhythmic network properties have been observed across recording techniques and task demands, but strong neuroscientific theories to situate such observations within a unified framework with direct relevance to explain neuropathologies remain scarce. Based on a comprehensive review of animal and human literature, we probe and introduce a neurophysiological framework to explain how coordinated cross-frequency and interregional oscillatory cortical dynamics underlie typical and atypical brain activation, and the formation of distributed functional ensembles supporting cortical networks underpinning perception and cognition. We propose that local regional activation by an external stimulus via a sensory pathway entails (1) attenuated alpha (8–14 Hz) and increased theta (4–8 Hz) and gamma (30–50 Hz) oscillatory activity, and (2) increased interactions among theta and gamma rhythms. These local dynamics also mediate the integration of activated neural populations into large-scale functional assemblies through neuronal synchronization. This comprehensive perspective into the animal and human literature indicates a further thinking beyond synchrony and connectivity and the readiness for more hypothesis-driven research and modeling toward unified principles of thalamo-cortical processing. We further introduced such a possible framework: “The ATG switch”. We also discussed evidence that alpha–theta–gamma dynamics emerging from thalamocortical interactions may be implicated and disrupted in numerous neurological and neuropsychiatric conditions.


Similar content being viewed by others
References
Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–39.
Ward LM. Synchronous neural oscillations and cognitive processes. Trends Cog Sci. 2003;7:553–9.
Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res. 2005;150:127–42.
Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolić D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Int Neurosci. 2009;3(17):1–19.
Canolty RT, Ganguly K, Kennerly SW, Cadieu CF, Koepsell K, Wallis JD, Carmena JM. Oscillatory phase coupling coordinates anatomically dispersed functional cell assemblies. Proc Natl Acad Sci USA. 2010;107:17356.
Llinás R, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–7.
Mazaheri A, Picton TW. EEG spectral dynamics during discrimination of auditory and visual targets. Cogn Brain Res. 2005;24:81–96.
Sauseng P, Klimesch W, Gruber WR, Birbaumer N. Cross-frequency phase synchronization: a brain mechanism of memory matching and attention. Neuroimage. 2008;40:308–17.
Doesburg SM, Green JJ, McDonald JJ, Ward LM. Rhythms of consciousness: binocular rivalry reveals large-scale oscillatory network dynamics mediating visual perception. PLoS ONE. 2009;4(7):e6142.
Holz EM, Glennon M, Prendergast K, Sauseng P. Theta–gamma phase synchronization during memory matching in visual working memory. Neuroimage. 2010;67:331–43.
Palva JM, Palva S. Discovering oscillatory interaction networks with M/EEG: challenges and breakthroughs. TICS. 2012;16:219–30.
Doesburg SM, Green JJ, McDonald JJ, Ward LM. Theta modulation of inter-regional gamma synchronization during auditory attention control. Brain Res. 2012;1431:77–85.
Kirschner A, Kam JWY, Handy TC, Ward LM. Differential synchronization in default and task-specific networks of the human brain. Front Hum Neurosci. 2012;6:139.
Burgess AP. Towards a unified understanding of event-related changes in the EEG: the firefly model of synchronization through cross frequency phase modulation. PLoS ONE. 2012;7(9):e45630. doi:10.1371/journal.pone.0045630.
FitzGerald THB, Valentin A, Selway R, Richardson MP. Cross-frequency coupling within and between the human thalamus and neocortex. Front Hum Neurosci. 2013;7:1–13.
Doesburg SM, Ward LM, Ribary U. The alpha–theta–gamma (ATG) switch: toward unified principles of cortical processing. Curr Trends Neurol. 2015;9:1–12.
Von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophsiol. 2000;38:301–13.
Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition timing hypothesis. Brian Res Rev. 2007;53:63–88.
Pfurtscheller G, Stancak A, Neuper C. Event-related synchronization (ERS) in the alpha-band—an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol. 1996;24:39–46.
Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by α-band EEG synchronization. Eur J Neurosci. 2007;25:603–10.
Hanslmayer S, Gross J, Klimesch W, Shapiro KL. The role of alpha oscillations in temporal attention. Brain Res Rev. 2011;67:331–43.
Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci USA. 2011;108:19377–82.
Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci USA. 2010;107:7580–5.
Doesburg SM, Ribary U, Herdman AT, Miller SP, Poskitt KJ, Moiseev A, Whitfield MF, Synnes A, Grunau RE. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage. 2011;54:2330–9.
Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:1–8.
Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–24.
Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JPR, Lado F, Mogilner A, Llinas R. Magnetic field tomography (MFT) of coherent thalamo-cortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA. 1991;88:11037–41.
Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced g-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–54.
Fell J, Klaver P, Elger CE, Fernández G. Suppression of EEG gamma activity may cause the attentional blink. Conscious Cogn. 2002;11:114–22.
Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–24.
Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–44.
Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev. 2010;34:739–44.
Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4726–36.
Osipova D, Takashima A, Oostenveld R, Fernandez G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci. 2006;26:7523–31.
Ribary U, Doesburg SM, Ward LM. Thalamocortical network dynamics: a framework for typical/atypical cortical oscillations and connectivity. In: Supek S, Aine CJ, editors. Magnetoencephalography—from signals to dynamic cortical networks. Heidelberg: Springer; 2014. p. 429–50.
Llinas R, Ribary U, Joliot M, Wang XJ. Content and context in temporal thalamocortical binding. In: Buzsaki G, Llinas R, Singer W, Berthoz A, Christen Y, editors. Temporal coding in the brain. Heidelberg: Springer; 1994. p. 251–72.
Llinás R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;90:2078–81.
Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601.
Llinás R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond. 1998;353:1841–9.
Ward LM. The thalamic dynamic core theory of conscious experience. Conscious Cogn. 2011;20:464–86.
Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504.
Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H. Long-range–projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–10.
Zilles K, Amunts K. Segregation and wiring in the brain. Science. 2012;335:1582–4.
Proske JH, Jeanmonod D, Verschure PFMJ. A computational model of thalamocortical dysrhythmia. Eur J Neurosci. 2011;. doi:10.1111/j.1460-9568.2010.07588.x.
Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus: common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996;119:363–75.
Sarnthein J, Jeanmonod D. High thalamocortical coherence in patients with Parkinson’s disease. J Neurosci. 2007;27:124–31.
Sarnthein J, Jeanmonod D. High thalamocortical coherence in patients with neurogenic pain. NeuroImage. 2008;39:1910–7.
De Ridder D, Vanneste S, Langguth B, Llinas R. Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front Neurol. 2015;6:124.
Lashley KS. The problem of serial order in behavior. In: Jeffress LA, editor. Cerebral mechanisms in behavior. New York: Wiley; 1951.
Ward LM. Dynamical cognitive science. Cambridge: MIT Press; 2002.
Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cog Sci. 2014;18:16–25.
Jensen O, Gips B, Bergmann TO, Bonnefond M. Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 2014;37:357–69.
Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O. Layer-specific entrainment of gamma-band neural activity by the alpha rhythm in monkey visual cortex. Curr Biol. 2012;22:2313–8.
Acknowledgements
This work was facilitated by funding from the BC Leading Edge Endowment Fund (BC LEEF) to UR, the Canadian Foundation for Innovation (CFI, CFI-IOF) relating to the Behavioral and Cognitive Neuroscience Institute (BCNI) to UR; from the Canadian Institutes of Health Research (CIHR: MOP-136935) and the Natural Sciences and Engineering Research Council (NSERC) of Canada (RGPIN-435659) to SMD; and from NSERC (A9958) to LMW. We thank Drs. Mazaheri, Picton, Burgess and Canolty for their generous permission to use modified parts of figures from their papers [5, 7, 14].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ribary, U., Doesburg, S.M. & Ward, L.M. Unified principles of thalamo-cortical processing: the neural switch. Biomed. Eng. Lett. 7, 229–235 (2017). https://doi.org/10.1007/s13534-017-0033-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-017-0033-4