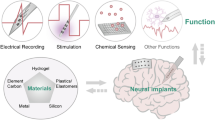
Abstract
Polymers are increasingly being used in implantable biomedical applications owing to their flexibility and compatibility with micro-fabrication processes. A liquid crystal polymer (LCP) is an inert, highly water-resistant polymer that is suitable for the encapsulation of electronic components and as a substrate material for fabricating neural interfaces. Therefore, the monolithic integration of a neural interface and electronics packaging is enabled by the use of an LCP, which has salient benefits in terms of performance and reliability. For these reasons, LCPs have been studied extensively as a base material for neural prosthetic devices. In this paper, we review recently developed enabling technologies, and demonstrate prototype devices and their performance capabilities. Lifetime estimations and technical challenges of LCP-based neural prosthetic devices are also described.
Similar content being viewed by others
References
Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008; 10: 275–309.
Khanna VK. Implantable medical electronics: prosthetics, drug delivery, and health monitoring. 1st ed. Cham: Springer International Publishing; 2016.
Prochazka A, Mushahwar VK, McCreery DB. Neural prostheses. J Physiol. 2001; 533: 99–109.
Stieglitz T, Schuetter M, Koch KP. Implantable biomedical microsystems for neural prostheses. IEEE Eng Med Biol Mag. 2005; 24(5): 58–65.
Teo AJT, Mishra A, Park I, Kim Y-J, Park W-T, Yoon Y-J. Polymeric biomaterials for medical implants and devices. ACS Biomater Sci Eng. 2016; 2(4): 454–72.
Wise KD, Sodagar AM, Yao Y, Gulari MN, Perlin GE, Najafi K. Microelectrodes, microelectronics, and implantable neural microsystems. P IEEE. 2008; 96(7): 1184–202.
Jiang G, Zhou DD. Technology advances and challenges in hermetic packaging for implantable medical devices. In: Zhou DD, Greenbaum E, editors. Implantable neural prostheses 2. Biological and medical physics, biomedical engineering. London: Springer; 2010. pp. 27–61.
Zhou DD, Greenberg RJ. Microelectronic visual prostheses. In: Greenbaum E, Zhou DD, editors. Implantable neural prostheses 1: Devices and applications. New York: Springer; 2009. pp. 1–42.
Brown KD, Balkany TJ. Benefits of bilateral cochlear implantation: a review. Curr Opin Otolaryngol Head Neck Surg. 2007; 15(5): 315–8.
Gantz BJ, Mccabe BF, Tyler RS. Use of multichannel cochlear implants in obstructed and obliterated cochleas. Otolaryngol Head Neck Surg. 1988; 98(1): 72–81.
Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991; 352(6332): 236–8.
Zeng F-G, Rebscher SJ, Fu Q-J, Chen H, Sun X, Yin L, Ping L, Feng H, Yang S, Gong S, Yang B, Kang HY, Gao N, Chi F. Development and evaluation of the Nurotron 26-electrode cochlear implant system. Hear Res. 2015; 322: 188–99.
Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel J-A, Stanga PE, Cideciyan AV, Duncan JL, Eliott D, Filley E, Ho AC, Santos A, Safran AB, Arditi A, Del Priore LV, Greenberg RJ. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology. 2012; 119(4): 779–88.
Rizzo JF, Wyatt J. Review: prospects for a visual prosthesis. Neuroscientist. 1997; 3(4): 251–62.
Schmidt E, Bak M, Hambrecht F, Kufta C, O’rourke D, Vallabhanath P. Feasibility of a visual prosthesis for the blind based on intracortical micro stimulation of the visual cortex. Brain. 1996; 119: 507–22.
Shepherd RK, Shivdasani MN, Nayagam DAX, Williams CE, Blamey PJ. Visual prostheses for the blind. Trends Biotechnol. 2013; 31: 562–71.
Veraart C, Wanet-Defalque M-C, Gérard B, Vanlierde A, Delbeke J. Pattern recognition with the optic nerve visual prosthesis. Artif Organs. 2003; 27(11): 996–1004.
Kern DS, Kumar R. Deep brain stimulation. Neurologist. 2007; 13(5): 237–52.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005; 45(5): 651–60.
Perlmutter JS, Mink JW. Deep brain stimulation. Annu rev neurosci. 2006; 29: 229–57.
Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999; 284(5423): 2177–81.
Di Francesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991; 351(6322): 145–7.
An SK, Park S-I, Jun SB, Lee CJ, Byun KM, Sung JH, Wilson BS, Rebscher SJ, Oh SH, Kim SJ. Design for a simplified cochlear implant system. IEEE Trans Biomed Eng. 2007; 54: 973–82.
Testerman RL, Rise MT, Stypulkowski PH. Electrical stimulation as therapy for neurological disorders. IEEE Eng Med Biol. 2006; 25(5): 74–8.
Weiland JD, Liu W, Humayun MS. Retinal prosthesis. Annu Rev Biomed Eng. 2005; 7: 361–401.
Castagnola V, Descamps E, Lecestre A, Dahan L, Remaud J, Nowak LG, Bergaud C. Parylene-based flexible neural probes with PEDOT coated surface for brain stimulation and recording. Biosens Bioelectron. 2015; 67: 450–7.
Cho S-H, Lu HM, Cauller L, Romero-Ortega MI, Lee J-B, Hughes GA. Biocompatible SU-8-based microprobes for recording neural spike signals from regenerated peripheral nerve fibers. IEEE Sens J. 2008; 8(11): 1830–6.
Hassler C, von Metzen RP, Ruther P, Stieglitz T. Characterization of parylene C as an encapsulation material for implanted neural prostheses. J Biomed Mater Res B. 2010; 93(1): 266–74.
Normann RA, Maynard EM, Rousche PJ, Warren DJ. A neural interface for a cortical vision prosthesis. Vision Res. 1999; 39(15): 2577–87.
Rodger DC, Weiland JD, Humayun MS, Tai Y-C. Scalable high lead-count parylene package for retinal prostheses. Sensor Actuat B-Chem. 2006; 117: 107–14.
Rodri FJ, Ceballos D, Schu M, Valero A, Valderrama E, Stieglitz T, Navarro X. Polyimide cuff electrodes for peripheral nerve stimulation. J Neurosci Methods. 2000; 98(2): 105–18.
Rousche PJ, Pellinen DS, Pivin Jr DP, Williams JC, Vetter RJ, Kipke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans Biomed Eng. 2001; 48(3): 361–71.
Weiland JD, Humayun MS, Eckhardt H, Ufer S, Laude L, Basinger B, Tai Y-C. A comparison of retinal prosthesis electrode array substrate materials. Conf Proc IEEE Eng Med Biol Soc. 2009; 4140–3.
Hassler C, Boretius T, Stieglitz T. Polymers for neural implants. J Polym Sci Pol Phys. 2011; 49(1): 18–33.
Kim BJ, Meng E. Review of polymer MEMS micromachining. J Micromech Microeng. 2015; 26(1): 013001.
Kim ET, Seo J-M, Woo S, Zhou J, Chung H, Kim S. Fabrication of pillar shaped electrode arrays for artificial retinal implants. Sensors. 2008; 8(9): 5845–56.
Kim ET, Kim C, Lee SW, Seo J-M, Chung H, Kim SJ. Feasibility of microelectrode array (MEA) based on siliconepolyimide hybrid for retina prosthesis. Invest Ophthalmol Vis Sci. 2009; 50(9): 4337–41.
Loeb GE, Peck RA. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J Neurosci Methods. 1996; 64(1): 95–103.
Jeong J, Lee SW, Min KS, Shin S, Jun SB, Kim SJ. Liquid crystal polymer (LCP), an attractive substrate for retinal implant. Sensor Mater. 2012; 24(4): 189–203.
Jeong J, Shin S, Lee GJ, Gwon TM, Park JH, Kim SJ. Advancements in fabrication process of microelectrode array for a retinal prosthesis using liquid crystal polymer (LCP). Conf Proc IEEE Eng Med Biol Soc. 2013; 5295–8.
Lee SW, Min KS, Jeong J, Kim J, Kim SJ. Monolithic encapsulation of implantable neuroprosthetic devices using liquid crystal polymers. IEEE T Biomed Eng. 2011; 58(8): 2255–63.
Jeong J, Bae SH, Min KS, Seo JM, Chung H, Kim SJ. A miniaturized, eye-conformable, and long-term reliable retinal prosthesis using monolithic fabrication of liquid crystal polymer (LCP). IEEE T Biomed Eng. 2015; 62(3): 982–9.
Gwon TM, Min KS, Kim JH, Oh SH, Lee HS, Park M-H, Oh SH, Kim SJ. Fabrication and evaluation of an improved polymer-based cochlear electrode array for atraumatic insertion. Biomed Microdevices. 2015; 17(2): 32.
Lee SE, Jun SB, Lee HJ, Kim J, Lee SW, Im C, Shin H-C, Chang JW, Kim SJ. A flexible depth probe using liquid crystal polymer. IEEE T Biomed Eng. 2012; 59(7): 2085–94.
Min KS, Oh SH, Park M-H, Jeong J, Kim SJ. A polymer-based multichannel cochlear electrode array. Otol Neurotol. 2014; 35(7): 1179–86.
Shin S, Kim J, Jeong Jeong J, Gwon TM, Choi GJ, Lee SE, Kim J, Jun SB, Chang JW, Kim SJ. High charge storage capacity electrodeposited iridium oxide film on liquid crystal polymer-based neural electrodes. Sensor Mater. 2016; 28(3): 243–60.
Wang K, ast, Liu CC, Durand DM. Flexible nerve stimulation electrode with iridium oxide sputtered on liquid crystal polymer. IEEE T Biomed Eng. 2009; 56(1): 6–14.
Min KS, Lee CJ, Jun SB, Kim J, Lee SE, Shin J, Chang JW, Kim SJ. A liquid crystal polymer-based neuromodulation system: an application on animal model of neuropathic pain. Neuromodulation. 2014; 17(2): 160–9.
Na SI, Kim S, Kim T, Lee H, Lee H, Kim S. A 32-channel neural recording system with a liquid-crystal polymer MEA. Conf Proc IEEE Biomed Circuits Syst Conf. 2013; 13–6.
Lee CJ, Oh SJ, Song JK, Kim SJ. Neural signal recording using microelectrode arrays fabricated on liquid crystal polymer material. Mater Sci Eng: C. 2004; 24(1-2): 265–8.
Bae SH, Che J-H, Seo J-M, Jeong J, Kim ET, Lee SW, Koo K, Suaning GJ, Lovell NH, Cho DI, KimSJ, Chung H. In vitro biocompatibility of various polymer-based microelectrode arrays for retinal prosthesis. Investig Ophthalmol Vis Sci. 2012; 53(6): 2653–7.
Gwon TM, Kim JH, Choi GJ, Kim SJ. Mechanical interlocking to improve metal–polymer adhesion in polymerbased neural electrodes and its impact on device reliability. J Mater Sci. 2016; 51(14): 6897–912.
Jeong J, Bae SH, Seo J-M, Chung H, Kim SJ. Long-term evaluation of a liquid crystal polymer (LCP)-based retinal prosthesis. J Neural Eng. 2016; 13:025004.
Hwang G-T, Im D, Lee SE, Lee J, Koo M, Park SY, Kim S, Yang K, Kim SJ, Lee K, Lee KJ. In vivo silicon-based flexible radio frequency integrated circuits monolithically encapsulated with biocompatible liquid crystal polymers. ACS Nano. 2013; 7(5): 4545–53.
Inagaki N. Role of polymer chain end groups in plasma modification for surface metallization of polymeric materials. Polym Int. 2009; 58(6): 585–93.
Qin Y, Howlader MMR, Deen MJ, Haddara YM, Selvaganapathy PR. Polymer integration for packaging of implantable sensors. Sensor Actuat B. 2014; 202: 758–78.
Matweb. http://www.matweb.com/. Accessed 22 June 2016.
Chlebowski AL. Advanced radio frequency materials for packaging of implantable biomedical devices. Master’s thesis; Purdue University; United States of America; 2009.
International Organization for Standardization. ISO 10993-5. In: Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. Geneve: ISO; 2009.
Lee SW, Seo J-M, Ha S, Kim ET, Chung H, Kim SJ. Development of microelectrode arrays for artificial retinal implants using liquid crystal polymers. Invest Ophthalmol Vis Sci. 2009; 50(12): 5859–66.
Chen L, Crnic M, Zonghe L, Johan L. Process development and adhesion behavior of electroless copper on liquid crystal polymer (LCP) for electronic packaging application. IEEE T Electron Pack M. 2002; 25(4): 273–8.
Dean JRN, Weller J, Bozack MJ, Rodekohr CL, Farrell B, Jauniskis L, Ting J, Edell D, Hetke J. Realization of ultra fine pitch traces on LCP substrates. IEEE T Compon Pack T. 2008; 31(2): 315–21.
Howlader MMR, Doyle TE. Low temperature nanointegration for emerging biomedical applications. Microelectron Reliab. 2012; 52: 361–74.
Kim W-S, Yun I-H, Lee J-J, Jung H-T. Evaluation of mechanical interlock effect on adhesion strength of polymer–metal interfaces using micro-patterned surface topography. Int J Adhes Adhes. 2010; 30(6): 408–17.
Min KS. A study on the liquid crystal polymer-based intracochlear electrode array. Doctoral thesis; Seoul National University. 2014.
Jeong J, Lee SW, Min K, Eom K, Bae SH, Kim SJ. Eyesurface conformable telemetric structure for polymer-based retinal prosthesis. Conf Proc IEEE Eng Med Biol Soc. 2011; 1097–100.
Jeong J, Lee SW, Min KS, Kim SJ. A novel multilayered planar coil based on biocompatible liquid crystal polymer for chronic implantation. Sensor Actuat A-Phys. 2013; 197: 38–46.
Cheung K, Gun L, Djupsund K, Yang D, Lee LP. A new neural probe using SOI wafers with topological interlocking mechanisms. Conf Proc Microtechnol Med Biol. 2000; 507–11.
Oh SJ, Song JK, Kim SJ. Neural interface with a silicon neural probe in the advancement of microtechnology. Biotechnol Bioproc E. 2003; 8(4): 252–6.
Peter N, Maria K, Aliette M, Ken Y, Ulrich GH. A 32-site neural recording probe fabricated by DRIE of SOI substrates. J Micromech Microeng. 2002; 12(4): 414–9.
Qing B, Wise KD. Single-unit neural recording with active microelectrode arrays. IEEE T Biomed Eng. 2001; 48(8): 911–20.
Shoji T, Takafumi S, Kunihiko M, Hiroyuki F. 3D flexible multichannel neural probe array. J Micromech Microeng. 2004; 14: 104–7.
Takeuchi S, Yoshida Y, Ziegler D, Mabuchi K, Suzuki T. Parylene flexible neural probe with micro fluidic channel. Conf Proc IEEE Int Conf MEMS. 2004; 208–11.
Farrell B, Jauniskis L, Phely-Bobin T, Streeter R, Edell D, Dean R. A polymer-based chronic nerve interface microelectrode array. Conf Proc Mater Res Soc. 2006; 0926-CC06-03.
Kang K, Choi IS, Nam Y. A biofunctionalization scheme for neural interfaces using polydopamine polymer. Biomaterials. 2011; 32(27): 6374–80.
Ajayi F, Garnham C, O’Donoghue GM. Pediatric experience of the reliability of the nucleus mini 22-channel cochlear implant. Am J Otol. 1997; 18(6):S44–5.
Rebscher SJ, Hetherington A, Bonham B, Wardrop P, Whinney D, Leake PA. Considerations for design of future cochlear implant electrode arrays: electrode array stiffness, size, and depth of insertion. J Rehabil Res Dev. 2008; 45(5): 731–47.
Spelman FA. Cochlear electrode arrays: past, present and future. Audiol Neuro-Otol. 2006; 11(2): 77–85.
Wang J, Wise KD. A hybrid electrode array with built-in position sensors for an implantable MEMS-based cochlear prosthesis. J Microelectromech S. 2008; 17(5): 1187–94.
Wang J, Wise KD. A thin-tilm cochlear electrode array with integrated position sensing. J Microelectromech S. 2009; 18(2): 385–95.
Wise KD, Bhatti PT, Wang JB, Friedrich CR. High-density cochlear implants with position sensing and control. Hearing Res. 2008; 242(1-2): 22–30.
Corbett S, Ketterl J, Johnson T. Polymer-based microelectrode arrays. Conf Proc Mater Res Soc. 2006; 0926-CC06-02.
Kim JH, Min KS, An SK, Jeong JS, Jun SB, Cho MH, Son YD, Cho ZH, Kim SJ. Magnetic resonance imaging compatibility of the polymer-based cochlear implant. Clin Exp Otorhinolaryngol. 2012; 5:S19–S23.
Fujikado T, Kamei M, Sakaguchi H, Kanda H, Morimoto T, Ikuno Y, Nishida K, Kishima H, Maruo T, Konoma K, Ozawa M, Nishida K. Testing of semichronically implanted retinal prosthesis by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011; 52(7): 4726–33.
Klauke S, Goertz M, Rein S, Hoehl D, Thomas U, Eckhorn R, Bremmer F, Wachtler T. Stimulation with a wireless intraocular epiretinal implant elicits visual percepts in blind humans. Invest Ophthalmol Vis Sci. 2011; 52(1): 449–55.
Stingl K, Bartz-Schmidt KU, Besch D, Braun A, Bruckmann A, Gekeler F, Greppmaier U, Hipp S, Hörtdörfer G, Kernstock C, Koitschev A, Kusnyerik A, Sachs H, Schatz A, Stingl KT, Peters T, Wilhelm B, Zrenner E. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Conf Proc Roy Soc B-Biol Sci. 2013; 280.
Park JH, Jeong J, Moon H, Kim C, Kim SJ. Feasibility of LCP as an encapsulating material for photodiode-based retinal implants. IEEE Photonics Tech L. 2016; 28(9): 1018–21.
Sundaram V, Sukumaran V, Cato ME, Liu F, Tummala R, Weiland JD, Nasiatka PJ, Tanguay AR. High density electrical interconnections in liquid crystal polymer (LCP) substrates for retinal and neural prosthesis applications. Conf Proc IEEE Electron Compon Tech. 2011; 1308–13.
Calhoun A, Peacock AJ. Polymer chemistry: properties and applications. Munich: Carl Hanser Verlag; 2006.
Greenhouse H, Lowry RK, Romenesko B. Hermeticity of electronic packages. Oxford: Elsevier; 2011.
Pham A-V. Packaging with liquid crystal polymer. IEEE Microw Mag. 2011; 12(5): 83–91.
Costello S, Desmulliez MPY, McCracken S. Review of test methods used for the measurement of hermeticity in packages containing small cavities. IEEE T Compon Pack Tech. 2012; 2: 430–8.
Aihara K, Chen MJ, Chen C, Pham A-VH. Reliability of liquid crystal polymer air cavity packaging. IEEE T Compon Pack Tech. 2012; 2(2): 224–30.
Ordonez JS, Boehler C, Schuettler M, Stieglitz T. Silicone rubber and thin-film polyimide for hybrid neural interfaces—a MEMS-based adhesion promotion technique. Conf Proc IEEE Eng Med Biol Soc Neural Eng. 2013; 872–5.
Ordonez JS, Boehler C, Schuettler M, Stieglitz T. Improved polyimide thin-film electrodes for neural implants. Conf Proc IEEE Eng Med Biol Soc. 2012; 5134–7.
Chang JHC, Yang L, Dongyang K, Yu-Chong T. Reliable packaging for parylene-based flexible retinal implant. Conf Proc Solid-State Sensor Actuat Microsyst. 2013; 2612–5.
von Metzen R, Stieglitz T. The effects of annealing on mechanical, chemical, and physical properties and structural stability of parylene C. Biomed Microdevices. 2013; 15(5): 727–35.
Leng A, Streckel H, Hofmann K, Stratmann M. The delamination of polymeric coatings from steel Part 3: effect of the oxygen partial pressure on the delamination reaction and current distribution at the metal/polymer interface. Corrosion Sci. 1998; 41(3): 599–620.
Leng A, Streckel H, Stratmann M. The delamination of polymeric coatings from steel. Part 2: first stage of delamination, effect of type and concentration of cations on delamination, chemical analysis of the interface. Corrosion Sci. 1998; 41(3): 579–97.
Aihara K, Chen MJ, Pham AV. Development of thin-film liquid-crystal-polymer surface-mount packages for band applications. IEEE T Microw Theory. 2008; 56(9): 2111–7.
Huang W, Wang X, Sheng M, Xu L, Stubhan F, Luo L, Feng T, Wang X, Zhang F, Zou S. Low temperature PECVD SiNx films applied in OLED packaging. Mater Sci Eng-B. 2003; 98(3): 248–54.
Reinke M, Kuzminykh Y, Hoffmann P. Low temperature chemical vapor deposition using atomic layer deposition chemistry. Chem Mater. 2015; 27(5): 1604–11.
Blahak C, Capelle HH, Baezner H, Kinfe TM, Hennerici MG, Krauss JK. Battery lifetime in pallidal deep brain stimulation for dystonia. Eur J Neurol. 2011; 18(6): 872–5.
Rubehn B, Stieglitz T. In vitro evaluation of the long-term stability of polyimide as a material for neural implants. Biomaterials. 2010; 31(13): 3449–58.
Spieth S, Brett O, Seidl K, Aarts AAA, Erismis MA, Herwik S, Trenkle F, Tätzner S, Auber J, Daub M, Neves HP, Puers R, Paul O, Ruther P, Zengerle R. A floating 3D silicon microprobe array for neural drug delivery compatible with electrical recording. J Micromech Microeng. 2011; 21(12): 125001.
Abidian MR, Martin DC. Multifunctional nanobiomaterials for neural interfaces. Adv Funct Mater. 2009; 19(4): 573–85.
Heo DN, Song S-J, Kim H-J, Lee YJ, Ko W-K, Lee SJ, Lee D, Park SJ, Zhang LG, Kang JY, Do SH, Lee SH, Kwon IK. Multifunctional hydrogel coatings on the surface of neural cuff electrode for improving electrode-nerve tissue interfaces. Acta Biomater. 2016; 39: 25–33.
Pellinen DS, Moon T, Vetter RJ, Miriani R, Kipke DR. Multifunctional flexible parylene-based intracortical microelectrodes. Conf Proc IEEE Eng Med Biol Soc. 2005; 5: 5272–5.
Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotechnol. 2015; 33(3): 277–84.
Huang W-C, Lai H-Y, Kuo L-W, Liao C-H, Chang P-H, Liu TC, Chen S-Y, Chen Y-Y. Multifunctional 3D patternable drug-embedded nanocarrier-based interfaces to enhance signal recording and reduce neuron degeneration in neural implantation. Adv Mater. 2015; 27(28): 4186–93.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gwon, T.M., Kim, C., Shin, S. et al. Liquid crystal polymer (LCP)-based neural prosthetic devices. Biomed. Eng. Lett. 6, 148–163 (2016). https://doi.org/10.1007/s13534-016-0229-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-016-0229-z