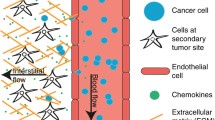
Abstract
Metastases many a time leave cancer patients untreatable and are one of the leading causes of death worldwide. Microfluidic platforms are arguably the most suitable for the study of cancer metastasis given its ability to mimic in vivo microenvironment of cancer tumor by manipulating its mechanical properties. This review discusses some applications of microfluidic platforms and their advantages for cancer biology and pathology. Studies of cancer metastasis conducted on its compositional steps enable us to elucidate elementary mechanisms through disease modeling. From that, communication and interaction of cancer cells, cellular metabolism related issues, and ultimately cancer drug discovery and delivery are manipulated on microfluidic platforms.
Similar content being viewed by others
Abbreviations
- 2D:
-
two-dimensional
- 3D:
-
three-dimensional
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- HEC:
-
Human endothelial cell
- hEGC:
-
Human embryonic germ cell
- VEGF:
-
Vascular endothelial growth factor
References
Boyle P, Levin B. World cancer report. IARC Sci Publ. 2008; 9.
Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002; 2(8):563–572.
Paweletz, CP, Charboneau L, Liotta LA. Overview of metastasis assays. Curr Protoc Cell Biol. 2001; 19.1.1–9. doi: 10.1002/0471143030.cb1901s12
Wang R, Xu J, Juliette L, Castileja A, Love J, Sung SY, Zhau HE, Goodwin TJ, Chung LWK. Three-dimensional co-culture models to study prostate cancer growth, progression and metastasis to bone. Semin Cancer Biol. 2005; 15:353–364.
Keese CR, Bhawe K, Wegner J, Giaever I. Real time impedance assay to follow the invasive activities of metastatic cells in culture. BioTechniques. 2002; 33:842–850.
Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962; 115:453–466.
Woodward JKL, Nichols CE, Rennie IG, Parsons MA, Murray AK, Sisley K. An in vitro assay to assess uveal melanoma invasion across endothelial and basement membrane barriers. Invest Ophth Vis Sci. 2002; 43:1708–1714.
Li Y, Zhu C. A modified Boyden chamber assay for tumor cell transendothelial migration in vitro. Clin Exp Metastas. 1999; 17(5):423–429.
Albini A, Benelli R, Noonan DM, Brigati C. The “Chemoinvasion assay”: a tool to study tumor and endothelial cell invasion of basement membranes. Int J Dev Biol. 48:563–571.
Shaw LM. Tumor cell invasion assays. Methods Mol Biol. 2005; 294:97–105.
Woo MMM, Salamanca CM, Minor A, Auersperg N. An improved assay to quantitate the invasiveness of cells in modified boyden chambers. In Vitro Cell Dev-An. 2007; 43(1):7–9
Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003; 24(24):4337–4351.
Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007; 13(10):3563–3576.
Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001; 294(5547):1708–1712.
Jeong GS, Kwon GH, Kang AR, Jung BY, Park Y, Chung S, Lee SH. Microfluidic assay of endothelial cell migration in 3D interpenetrating polymer semi-network HA-Collagen hydrogel. Biomed Microdevices. 2011; 13(4):712–723.
Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, Kamm RD. Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J. 2009; 23(7):2155–2164.
Chung S, Sudo R, Zervantonakis IK, Rimchala T, Kamm RD. Surface-treatment-induced three-dimensional capillary morphogenesis in a microfluidic platform. Adv Mater. 2009; 21(47):4863–4867.
Kothapalli CR, van Veen E, de Valence S, Chung S, Zervantonakis Y, Gertler FB, Kamm RD. A high-throughput microfluidic assay to study axonal response to growth factor gradients. Lab Chip. 2011; 11(3):497–507.
Shin Y, Jeon J, Jeong GS, Han S, Shin S, Lee S-H, Sudo R, Kamm RD, Chung S. in vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip. 2011; 11(13):2175–2181.
Volder RD, Kong HJ. Biomaterials for studies in cellular mechanotransduction. Tools Exploring Mechanobiology. 267–277.
Beebe DJ, Mensing GA, Walker GM. Physics and applications of microfluidics in biology. Annu Rev Biomed Eng. 2002; 4:261–286.
Beebe DJ, Moore JS, Yu Q, Liu RH, Kraft ML, Jo BH, Devadoss C. Microfludic tectonics: a comprehensive construction platform for microfluidic systems. P Natl Acad Sci USA. 2000; 97:13488–13493.
Chung S, Sudo R, Vickerman V, Zervantonakis IK, Kamm RD. Microfluidic platform for studies of angiogenesis, cell migration, and cell-cell interactions. Ann Biomed Eng. 2010; 38:1164–1177.
Sahai Erik. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005; 15(1):87–95.
Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nature Reviews Molecular Cell Biology. 2006; 7:211–224.
Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011; 147:992–1009.
Hsu PP, Sabatini DM. Cancer cell metabolism: warburg and beyond. Cell. 2008; 134(5):703–707.
Locasale JW, Cantley LC. Altered metabolism in cancer. BMC Biology. 2010; 8:88.
Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007; 99:1583–1593.
Semino CE, Kamn RD, Lauffenburger DA. Autocrine EGF receptor activation mediates endothelial cell migration and vascular morphogenesis induced by VEGF under interstitial flow. Exp Cell Res. 2006; 312(3):289–298.
Song XY, Kong BH, Li D. A new tool for probing of cell-cell communication: human embryonic germ cells inducing apoptosis of SKOV3 ovarian cancer cells on a microfluidic chip. Biotechnol Lett. 2008; 30(9):1537–1543.
Hsu TH, Xiao JL, Tsao YW, Kao YL, Huang SH, Liao WY, Lee CH. Analysis of the paracrine loop between cancer cells and fibroblasts using a microfluidic chip. Lab Chip. 2011; 11:1808–1814.
Polacheck WJ, Charest JL, Kamn RD. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci. 2011; 108:11115–11120.
Haessler U, Teo JCM, Foretay D, Renaud P, Swartz MA. Migration dynamics of breast cancer cells in a tunable 3D interstitial flow chamber. Integr Biol. 2012; 4:401–409.
Jeong GS, Han SW, Shin YJ, Kwon GH, Kamn RD, Lee SH, Chung S. Sprouting angiogenesis under a chemical gradient regulated by interactions with an endothelial monolayer in a microfluidic platform. Anal Chem. 2011; 83:8454–9.17.
Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009; 9:269–275.
Walsh CL, Babin BM, Kasinskas TW, Foster JA, McGarry MJ, Forbes NS. A multipurpose microfluidic device designed to mimic microenvironment gradients and develop targeted cancer therapeutics. Lab Chip. 2009; 9(4):545–554.
Liu XH, Kirschenbaum A, Yao S, Stearns ME, Holland JF, Claffey K, Levine AC. Upregulation of vascular endothelial growth factor by cobalt chloride-simulated hypoxia is mediated by persistent induction of cyclooxygenase-2 in a metastatic human prostate cancer cell line. Clin Exp Metastas. 1999; 17:687–694.
Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-a and von hippellindau protein by direct binding to hypoxia-inducible factor-a. J Biol Chem. 2003; 278:15911–15916.
Webster A, Dyer CE, Haswell SJ, Greenman J. A microfluidic device for tissue biopsy culture and interrogation. Anal Meth. 2010; 2:1005–1007.
Duffy MJ, Crown J. A personalized approach to cancer treatment: how biomarkers can help. Clin Chem. 2008; 54(11):1770–1779.
Eccles SA, Bo C, Court W. Cell migration/invasion assays ad their application in cancer drug discovery. Biotech Annu Rev. 2005; 11:391–421.
Grist S, Yu LF, Chrostowski L, Cheung KC. Microfluidic cell culture systems with integrated sensors for drug screening. Proc of SPIE. 2012; 8251:825103–825112.
Wu LY, Carlo DD, Lee LP. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed Microdevices. 2008; 10:197–202.
LaVan DA, McGuire T. Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol. 2003; 21(10):1184–1191.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ng, J., Shin, Y. & Chung, S. Microfluidic platforms for the study of cancer metastasis. Biomed. Eng. Lett. 2, 72–77 (2012). https://doi.org/10.1007/s13534-012-0055-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-012-0055-x