Abstract
Stabilized and size-controlled gold nanoparticles were synthesized using molybdophosphoric acid (H3[PMo12O40], HPMo) and its vanadium-substituted mixed addenda (H3 + x [PMo12 − x V x O40], (x = 0–3); and HPMoVx) under UV irradiation by a simple green method. In the process, HPMo and HPMoV x play the role of photocatalyst, reducing agent and efficient stabilizer. Control of gold nanoparticles size was achieved by variation of initial gold ions concentration and molar ratio of HPMo to gold ions. The synthesis rate of Au nanoparticles was found to be parallel to an increasing x value in the order of: HPMoV3 > HPMoV2 > HPMoV > HPMo, which leads to smaller and more uniform particles. Also, by substitution of vanadium instead of molybdenum in the HPMo formula, the morphology of nanoparticles was gradually changed from spherical-shaped nanoparticles in the presence of HPMo to nanorods in the case of HPMoV3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the usual metal nanostructures is gold nanoparticles (Au NPs) which are used in optics, electrochemistry, catalysis, sensors, environmental engineering, and electronics because they are stable, relatively nontoxic, and biocompatible [1–5]. The size and shape of Au NPs strongly affect their physical and chemical properties and intense research has been devoted to the morphological control of these nanostructures in recent years. Finding the methods in which one could control the size and shape of the prepared nanoparticles is of great interest in order to maximize the particles efficiency [6].
A lot of techniques have been developed for the synthesis of these nanoparticles, such as electrochemical [7], chemical reduction [8], sonochemical [9], photochemical [10], and so on. To date, solution-based wet chemical synthesis is believed to be the best route to new nanostructures [11, 12]. In most of these procedures, the use of an organic environment and a relatively high temperature are common. Controlling size and shape of nanoparticles can be achieved through the control of nucleation and growth steps by varying synthesis parameters, including activity of reducing agents, type and concentration of precursors, and also nature and amount of protective agents [13–15]. However, the intervention of environmentally harmful and toxic chemicals in the Au NPs preparation procedures is inevitable.
Recently, the green synthesis or fabrication of Au nanostructures has been incomprehensively studied using harmless alternative polyoxometalates (POMs) [16–19]. Since the environmental care is one of the worldwide increasing worries, green chemistry has been defined as a set of principles which reduce or eliminate the use of hazardous substances or catalysts [20]. This fact encourages scientists to make efforts in finding processes working in this direction. For this reason, there is still a good scope for research towards finding green and ecofriendly materials, solvents, and catalysts in different reactions. Along this line, introducing clean processes and utilizing ecofriendly and green catalysts which can be simply recycled at the end of reactions have been under permanent attention and demands.
POMs as solid acid catalysts are green with respect to corrosiveness, safety, quantity of waste, recyclability, and separability. Therefore, using them in various processes is one of the innovative trends.
They are a unique class of molecularly defined inorganic metal–oxide clusters which have exceptional properties such as: strong Brönsted acidity, high hydrolytic stability (pH = 0–12), high thermal stability, and operation in pure water without any additive [21, 22]. Attractively, POM’s structures remain unchanged under stepwise and multielectron redox reactions and can be reduced by photochemical and electrochemical procedures using suitable reducing agents [23].
These compounds have been used as both reducing agents and stabilizers for the synthesis of metal nanoparticles such as Ag, Au, Pt, Se, and Pd upon illumination with UV/near-Vis light [6, 18, 24–28]. There are only limited reports regarding synthesis of gold nanoparticles (Au NPs) using these kinds of green materials. Troupis et al. used the photocatalytic process for Au NPs synthesis in the presence of H3[SiW12O40] [18]. Mandal et al. have synthesized more complicated nanostructures such as Au–Ag core–shell dimetallic compounds [29] and Au nanosheets [30]. Various crystalline gold nanostructures have also been synthesized using β-[H4PMo12O40]3− [12] and transition metal monosubstituted POMs (PW11MO40, M = Cu2+, Ni2+, Zn2+, Fe3+) [31]. Moreover, in our previous work, we have synthesized Au NPs using Preyssler acid with a simple photoreduction technique [32].
Although, some Keggin, mixed valence, and Preyssler types of POMs have been used in the synthesis of Au NPs, to the best of our knowledge, the role of molybdophosphoric acid, HPMo, and its vanadium-substituted mixed addenda, HPMoV x , has not been studied. HPMo, similar to the other types of POMs [18], can be reduced in the presence of oxidizable organic substrates like alcohols (e.g., propan-2-ol), under UV irradiation (Eq. (1)):
In contact with gold ions, [PMo12O40]4− is able to transfer electrons efficiently to gold ions and reduce them to Au0. The color of the solution is then gradually turned from colorless to pink indicating the formation of Au0. Equation (2) represents this reaction:
According to Eqs. (1) and (2), HPMo ions can be utilized cyclically as oxidizing or reducing agent and propan-2-ol plays the role of sacrificial agent.
In the present work, we have investigated the synthesis of gold nanostructures generated by a green chemistry-type process, using HPMo and HPMoV x in the absence of any surfactant or seed. Also, the effect of gold ion concentration or molar ratio of HPMo to gold ion was studied on the size of Au NPs. Besides, vanadium-substituted mixed addenda of HPMo (i.e., HPMoV x ) was used for the synthesis of Au NPs and the effect of addition of vanadium atom (x = 0–3) in the POMs structure was explored on the size and the shape of prepared NPs.
Experimental
Chemicals and apparatus
H3[PMo12O40] and other chemicals were purchased from Merck Company and used as received. H4[PMo11VO40], H5[PMo10V2O40] and H6[PMo9V3O40] were prepared according to the procedure reported in the literature [33]. UV-visible spectra were obtained using Avantes Avaspec-3648 single beam instrument. The synthesized Au NPs were characterized mainly by particle size distribution (PSD) using a ZetaSizer Nano ZS apparatus (Malvern Instruments Ltd.) as a laser particle sizer. The instrument allowed to measure particle size taking the advantage of optoelectronic systems. Also, nanoparticles were characterized using Transmission Electron Microscopy (Philips CM-120).
Synthesis procedure of Au NPs
In a typical experiment, 5.5 × 10−7 mol of HPMoVx was dissolved in 5 mL distilled water and then 10 mL HAuCl4 (5 × 10−4 M) and 2 mL propan-2-ol were added. The solution was placed into a spectrophotometer cell and deaerated with N2 gas. Then, the mixture was irradiated by UV light (125 W high pressure mercury vapor lamp) under continuous stirring. Reaction was performed in a constant room temperature, using water circulating around the cell. The color of the solution changed from colorless or pale yellow (at high HPMo concentration) to pink, indicating the formation of Au NPs. The nanoparticles were separated from the reaction mixture by a high-speed centrifuge (14,000 rpm), washed twice with water and redispersed in water before any analysis.
Results and discussion
Polyoxometalates, regarding their redox abilities, can be divided into two groups of mono-oxo (type I) and cis-dioxo (type II). This classification is based on the number of terminal oxygen atoms attached to each addenda atom, e.g., molybdenum or tungsten, in the polyanion. Examples of type I polyanions are Keggins, Wells–Dawsons, and their derivatives that have one terminal oxygen atom M=O per each addenda atom. Type II polyanions can be represented by the Dexter–Silverton anion which has two terminal oxygens in cis positions on each addenda atom.
In type I octahedral MO6, the lowest unoccupied molecular orbital (LUMO) is a nonbonding metal-centered orbital, whereas the LUMO for type II octahedral is antibonding with respect to the terminal M=O bonds. Consequently, type I polyoxometalates are reduced easily and often reversibly to form mixed-valence species, heteropoly blues, which can act as an oxidant. In contrast, type II polyoxometalates are reduced with more difficultly and irreversibly to complexes with yet unknown structures [34, 35]. For this reason, only type I heteropoly compounds, especially Keggins, are of interest for catalytic reactions. Therefore, H3[PMo12O40] with Keggin structure was selected since, to the best of our knowledge, the role of that has not been studied in the redox controlled synthesis of Au nanoparticles. Keeping in mind that the introduction of vanadium (V) into the Keggin framework is beneficial for redox catalysis [36] and also it can shift its reactivity from acid-dominated to redox-dominated, we selected H3 + x [PMo12 − x V x O40] (x = 1–3).
The process was monitored by the visible absorption spectrometry. Figure 1 shows the UV/Vis spectra of the mixture at different treatment stages. It can be seen that primary solution does not have any distinct absorption band in the wavelength range of 400–800 nm. But, after 35 min, the absorption bands were observed in the SPR band of gold NPs at about 535 nm. These absorption bands caused by the excitation of surface–plasmon vibrations indicate formation of Au NPs. Furthermore, until 20 min irradiation, there is no absorption band at 535 nm, indicating that no nanoparticles were formed. In this time interval, the rate of Au NPs production reaction (Eq. 2) is negligible and Eq. 1 is in progress. From the figure, it can be observed that by increasing the time, the absorption band becomes sharper and the resonance intensity enhances due to the formation of Au NPs during the process.
Besides the role as reducing agent, HPMo also plays the role of stabilizing agent in the above reactions. In our previous study, it was shown that in the absence of POMs, Au particles were precipitated in less than 2 days [32], but the resulting colloid in the presence of HPMo was stable without any precipitation for more than 3 months. It might be due to the adsorption of HPMo polyanions onto the surface of Au NPs which provide both steric stabilization and kinetic stabilization through coulombic repulsion between the negatively charged particles.
In the photolysis reaction, the propan-2-ol serves as a sacrificial agent for the photoformation of reduced HPMo, HPMo(e−), which reacts with gold ions to produce Au NPs. A control experiment was performed in which 2 mL propan-2-ol was added to the deaerated aqueous solution of HAuCl4 and irradiated for 6 h. There was no change in the color of solution and the characteristic gold absorption band was not observed. It indicates that the UV-irradiated propan-2-ol is not responsible for the reduction of Au3+. On the other hand, our observations show that the amount of propan-2-ol affects the reaction rate which influences the size and uniformity of the synthesized Au NPs and by increasing the amount of the propan-2-ol, smaller and more uniform nanoparticles were obtained [15].
The rate of gold ions reduction (Eq. 2) affects strongly the initial nucleation and final size of nanoparticles. In fact, in this reaction, size control of Au NPs can be achieved via rate control of Eq. 2 by changing the experimental conditions. Faster reduction of gold ions leads to formation of smaller and more uniform nanoparticles. The initial Au3+ ion concentration and also HPMo amount are two parameters which can influence the reaction rate in Eq. (2). Our findings show that increasing the initial Au3+ concentration enhances reaction rate. As indicated in Fig. 2, an increase in concentration of gold ions results in the formation of larger nanoparticles. Also, our observations have shown that at higher gold ion concentrations, the stability of the prepared Au NPs decreases and they are precipitated after a short time. It might be due to (1) their bigger size or (2) increasing [Au3+]/[HPMo] ratio in which the amount of HPMo might not be sufficient for Au NPs stabilization.
Also, we have found that the desired size of Au NPs can be achieved via changing the initial amount of HPMo. For this purpose, we have investigated the effect of [HPMo]/[Au3+] = γ on the size of synthesized gold NPs in which the initial concentration of Au3+ was kept constant (5 × 10−4 M). The results are shown in Fig. 3. At low value of γ, less than 0.73, the mean diameter of Au NPs was decreased by increasing the γ ratio. The fact that smaller Au NPs are formed with increasing the initial amount of HPMo implies that the nucleation process is enhanced more than the growth of nanoparticles.
Figure 3 also shows that by increasing the γ above 0.73, the size of the synthesized NPs exhibited a contrary trend and larger NPs were formed through increasing the HPMo amount. The reason of the opposing trend of large Au NPs might be due to higher coverage of HPMo polyanions on the exterior surface of Au NPs at higher HPMo value that inhibits the reaction rate in Eq. 2.
For the HPMo value of 5.5 × 10−6 M and 2 mL propan-2-ol, γ = 0.73 acts as a critical amount in the synthesis of Au NPs in our experimental condition. This value depends on the type of metal ions, POM type, propan-2-ol amount and other operating conditions (temp., pH, ionic strength, etc.). This behavior is similar to that found in many chemical reduction approaches to nanosystems, because the nucleation and growth sequences are both affected by the relative concentrations of the reducing agent and the precursor [28].
Effect of vanadium substitution in HPMo
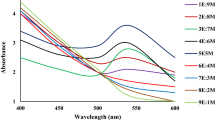
We have also investigated the effect of vanadium substitution in HPMo, i.e., HPMoV x (x = 1–3), on the photosynthesis rate of Au NPs as well as the morphology of nanoparticles. Figure 4 shows the surface plasmon resonance spectra of the synthesized Au NPs solutions in the presence of HPMoVx (x = 0–3) irradiated for 35 min. HPMoV x shift the peak of SPR bands gradually from 535 to 555 nm for x = 2, which indicates Au NPs become larger using HPMoV2.
When x = 3, a new peak appears at ~690 nm, which is related to the synthesis of Au nanorods [7]. In fact, nanorods show two plasmon bands commonly ascribed to light absorption (and scattering) along both the long axis (“longitudinal plasmon band”) and the short axis (“transverse plasmon band”) of the colloid particles. As the aspect ratio increases, the position of the longitudinal plasmon band red shifts, and the transverse plasmon band position stay relatively invariable at ~520 nm [37]. The appearance of the longitudinal plasmon band at ~690 indicates the preparation of nanorods with aspect ratio of ~2 [7, 35].
Moreover, they affect the rate of synthesis reactions presented in Eqs. (1) and (2) (see Fig. 5). Faster kinetics can be observed by increasing the number of vanadium atoms. Figure 5 also demonstrates that increasing the number of vanadium atom substituted in HPMo (x value) improve the redox potential of HPMo in the order of: HPMoV3 > HPMoV2 > HPMoV > HPMo. Moreover, the synthesis reaction rate of Au NPs as well as the nucleation rate is enhanced in the same order, but larger NPs are produced. This fact is shown in Fig. 6a–d. The PSD analysis in these cases, demonstrate just the approximate size of synthesized nanoparticles. It is clear that at the same reaction conditions, the mean diameters of synthesized Au NPs become 16.3, 19.6, 23.8, and 30, by changing x from 0 to 4, respectively.
Moreover, increasing x has an interesting effect on the shape of prepared nanoparticles. Figure 7a shows TEM image of Au NPs synthesized using HPMo. The nanoparticles are seen to be hexagonal and spherical in shape. By substituting a vanadium atom in HPMo, a few anisotropic and irregularly shaped structures are observed (Fig. 7b). Also, by increasing the number of vanadium atom in HPMo, Au nanorods are formed, and for x = 3 almost all hexagonal nanoparticles changed to nanorods. It has been also observed that all solutions including nanoparticles and nanorods are stable for few weeks. The TEM images confirm the PSD of the prepared Au nanoparticles using HPMo, but with increasing the x value in the formula, the PSD histograms underestimate the size of true particles. It may be due to the formation of inhomogeneous nanoparticles in the case of x = 2 and 3 and also nanorods in x = 3. In fact, the PSD is unable to show a real distribution of Au nanorods.
Conclusions
Molybdophosphoric acid and its vanadium-substituted products (HPMoV x , x = 0–3) were used as excellent photocatalysts, reducing agents, and stabilizers in the synthesis of gold nanoparticles. Uniform and size-controlled Au NPs were easily obtained by simple photolysis of HPMoV x /Au3+/propan-2-ol solution at room temperature. Controlling the size of nanoparticles was achieved by changing the rate of Au3+ reduction via variation of initial gold ions concentration and molar ratio of HPMo to gold ions. Faster reductions result in smaller and more uniform Au NPs as exhibited by increasing the initial concentration of gold ions or the value of x. It was found that 0.73 is a critical ratio for [HPMo]/[Au3+], in which for its lower range, increasing the ratio leads to the formation of smaller nanoparticles and for its higher value the opposite trend is happened. Our findings have shown that the reduction of Au3+ occurred in the order of: HPMoV3 > HPMoV2 > HPMoV > HPMo. It is suggested that, besides energy and composition of the LUMO, the presence of both Bronsted acidity and vanadium in the structure of mentioned heteropolyacids are responsible for catalytic activity. The greater protons number may lower the activation energy barrier, and the greater vanadium atoms may provide many sites for catalytic reaction. Also, by increasing the value of x in the HPMoV x formula, spherical nanoparticles changed to nanorods.
References
Ishida T, Haruta M (2007) Gold catalysts: towards sustainable chemistry. Angew Chem Int Ed 46(38):7154–7156
Castãneda MT, Merkoçi A, Pumera M, Alegret S (2007) Electrochemical genosensors for biomedical applications based on gold nanoparticles. Biosens Bioelectron 22(9–10):1961–1967
Rassaei L, Sillanpää M, French RW, Compton RG, Marken F (2008) Arsenite determination in the presence of phosphate at electro-aggregated gold nanoparticle deposits. Electroanalysis 20:1286–1292
Dubey S, Lahtinen M, Sillanpää M (2010) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45:1065–1071
Dubey S, Lahtinen M, Särkkä H, Sillanpää M (2010) Bioprospective of Sorbus acuparia leaf extract in development of silver and gold nanocolloids. Colloids and Surfaces B: Biointerfaces 80:26–33
Troupis A, Triantis T, Hiskia A, Papaconstantinou E (2008) Rate-redox-controlled size-selective synthesis of silver nanoparticles using polyoxometalates. Eur J Inorg Chem 2008(36):5579–5586
Yu Y-Y, Chang S-S, Lee C-L, Wang CRC (1997) Gold nanorods: electrochemical synthesis and optical properties. J Phys Chem B 101(34):6661–6664
Jana NR, Gearheart L, Murphy CJ (2001) Evidence for seed-mediated nucleation in the chemical reduction of gold salts to gold nanoparticles. Chem Mater 13(7):2313–2322
Caruso RA, Ashokkumar M, Grieser F (2002) Sonochemical formation of gold sols. Langmuir 18(21):7831–7836
Kim F, Song JH, Yang P (2002) Photochemical synthesis of gold nanorods. J Am Chem Soc 124(48):14316–14317
Caixia K, Zhu X, Guanghou W (2006) Single-crystalline gold microplates: synthesis, characterization, and thermal stability. J Phys Chem B 110(10):4651–4656
Zhang G, Keita B, Biboum RN, Miserque F, Berthet P, Dolbecq A, Mialane P, Catala L, Nadjo L (2009) Synthesis of various crystalline gold nanostructures in water: the polyoxometalate β-[H4PMo12O40]3- as the reducing and stabilizing agent. J Mater Chem 19:8639–8644
Chen Y, Liew KY, Li J (2008) Size-controlled synthesis of Ru nanoparticles by ethylene glycol reduction. Mater Lett 62:1018–1021
Hostetler JM, Wingate EJ, Zhong JC, Harris EJ, Vachet RW, Clark RM (1998) Langmuir 14:17
Ayati A, Ahmadpour A, Bamoharram FF, Heravi MM, Rashidi H, Tanhaei B (2011) Application of molybdophosphoric acid in size-controlled synthesis of gold nanoparticles under UV irradiation. Int J Nanosci Nanotech 7(2):87–93
Bamoharram FF, Ahmadpour A, Heravi MM, Ayati A, Rashidi H, Tanhaei B (2011) Recent advances in application of polyoxometalates for the synthesis of nanoparticles. Synth React Inorg Met Org Chem. doi:10.1080/15533174.2011.609849
Bamoharram FF (2011) Role of polyoxometalates as green compounds in recent developments of nanoscience. Synth React Inorg Met Org Chem 41(8):893–922
Troupis A, Hiskia A, Papaconstantinou E (2002) Synthesis of metal nanoparticles by using polyoxometalates as photocatalysts and stabilizers. Angew Chem Int Ed 41:1911–1913
Ayati A, Ahmadpour A, Bamoharram FF, Heravi MM, Rashidi H, Tanhaei B (2011) A new photocatalyst for preparation of silver nanoparticles and their photcatalysis of the decolorization of methyl orange. J Nanostruct Chem 2(1):15–22
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, New York
Papaconstantinou E (1989) Photochemistry of polyoxometallates of molybdenum and tungsten and/or vanadium. Chem Soc Rev 18:1–31
Wang BE, Hu WC, Xu L (1998) Introduction to polyacid chemistry. Chemical Industry Press, Beijing, p 87
Weinstock AI (1998) Homogeneous-phase electron-transfer reactions of polyoxometalates. Chem Rev 98(1):113–170
Laurent R, Claire C-C, Sébastien S, Isabelle L (2008) Photocatalytic reduction of Ag2SO4 by Dawson-derived sandwich complex. Macromol Symp 270(1):117–122
Mandal S, Das A, Srivastava R, Sastry M (2005) Keggin ion mediated synthesis of hydrophobized Pd nanoparticles for multifunctional catalysis. Langmuir 21(6):2408–2413
Troupis A, Gkika E, Hiskia A, Papaconstantinou E (2006) Photocatalytic reduction of metals using polyoxometallates: recovery of metals or synthesis of metal nanoparticles. Comptes Rendus Chimie 9(5–6):851–857
Yang L, Shen Y, Xie A, Zhang B (2007) Facile size-controlled synthesis of silver nanoparticles in UV-irradiated tungstosilicate acid solution. J Phys Chem C 111(14):5300–5308
Triantis T, Troupis A, Gkika E, Alexakos G, Boukos N, Papaconstantinou E, Hiskia A (2009) Photocatalytic synthesis of Se nanoparticles using polyoxometalates. Catal Today 144(1–2):2–6
Mandal S, Selvakannan RP, Pasricha R, Sastry M (2003) Keggin ions as UV-switchable reducing agents in the synthesis of Au Core-Ag shell nanoparticles. J Am Chem Soc 125(28):8440–8441
Sanyal A, Mandal S, Sastry M (2005) Synthesis and assembly of gold nanoparticles in quasi-linear lysine-Keggin-ion colloidal particles. Adv Funct Mater 15(2):273–280
Niu C, Wu Y, Wang Z, Li Z, Li R (2009) Synthesis and shapes of gold nanoparticles by using transition metal monosubstituted heteropolyanions as photocatalysts and stabilizers. Frontiers of Chemistry in China 4(1):44–47
Ayati A, Ahmadpour A, Bamoharram FF, Heravi MM, Rashidi H (2011) Photocatalytic synthesis of gold nanoparticles using preyssler acid and their photocatalytic activity. Chin J Catal 32(6):978–982
Heravi MM, Benmorad T, Bakhtiari K, Bamoharram FF, Oskooie HH (2007) H3+xPMo12-xVxO40 (heteropolyacids)-catalyzed regioselective nitration of phenol to o-nitrophenol in heterogeneous system. J Mol Catal A: Chem 264(1–2):318–321
Pope MT (1983) Heteropoly isopoly oxometalates. Springer, Berlin
Pope MT, Müller A (1991) Polyoxometalate chemistry: an old field with new dimensions in several disciplines. Angew Chem Int Ed Eng 30(1):34–48
Mizuno N, Misono M (1994) Heteropolyanions in catalysis. J Mol Catal 86(1–3):319–342
Murphy CA, Thompson LB, Chernak DJ, Yang JA, Sivapalan ST, Boulos SP, Huang J, Alkilany AM, Sisco PN (2011) Gold nanorod crystal growth: from seed mediated synthesis to nanoscale sculpting. Current Opinion Colloid Interface Sci 16:128–134
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ayati, A., Ahmadpour, A., Bamoharram, F.F. et al. Rate redox-controlled green photosynthesis of gold nanoparticles using H3 + x PMo12 − x V x O40 . Gold Bull 45, 145–151 (2012). https://doi.org/10.1007/s13404-012-0058-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-012-0058-5