Abstract
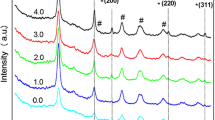
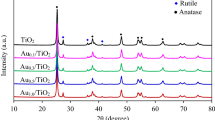
This work deals with the study of photodeposition (PD) of gold nanoparticles (AuNPs) on TiO2 by using different illumination sources, Medium pressure Mercury lamp (ML), Solar Simulator equipped with AM 1.5 (SL) and Tungsten lamp (WL). Different particle size of AuNPs on TiO2 were obtained by photodeposition method under different illumination sources, which clearly proves the influence of light source on the synthesis of Au–TiO2. The plasmonic activity of Au–TiO2 photocatalyst for water splitting reaction was observed to be strongly influenced by the particle size of Au as well as illumination source. Amongst the three different illumination sources used, smallest particle size for AuNP–TiO2 were observed under ML followed by SL and WL, as revealed by TEM analysis. Different illumination sources were also investigated to evaluate the activity of Au–TiO2 samples thus prepared under different illumination conditions. The order of hydrogen evolution rate (HER) observed for Au–TiO2 with different source of illuminations is ML > SL > WL. The highest HER of 1709 μmol/h was observed for Au–TiO2, which was synthesized and evaluated under ML irradiation. This may be explained on the basis of reduced catalytic activity and photothermal effect of Au nanoparticles with increasing particle size.
Similar content being viewed by others
References
K. Hashimoto, H. Irie, and A. Fujishima, Jpn. J. Appl. Phys. 44, 8269 (2005). doi 10.1143/JJAP.44.8269
X. Chen, S. Shen, L. Guo, and S. S. Mao, Chem. Rev. 110, 6503 (2010). doi 10.1021/crl001645
A. Fujishima and K. Honda, Nature 238, 37 (1972). doi 10.1038/238037a0
A. Fujishima, Jpn. Nanonet. Bull.
M. Ni, M. K. H Leung, D. Y. C. Leung, and K. Sumathy, Renew. Sustain. Energy Rev. 11, 401 (2007). doi 10.1016/j.rser.2005.01.009
W. Choi, A. Termin, and M. R. Hoffmann, J. Phys. Chem. 98, 13669 (1994). doi 10.1021/jl00102a038
D. Dvoranova, V. Brezova, M. Mazúr, and A. Malati, Appl. Catal. B: Environ. 37, 91 (2002). doi 10.1016/S0926-3373(01)00335-6
X.-Z. Shen, Z.-C. Liu, S.-M. Xie, and J. Guo, J. Hazard. Mater. 162, 1193 (2009). doi 10.1016/j.jhazmat.2008.06.004
H. Irie, Y. Watanabe, and K. Hashimoto, J. Phys. Chem. B 107, 5483 (2003). doi 10.1021/jp030133h
T.-H. Xu, C.-L. Song, Y. Liu, and G.-R. Han, J. Zhejiang Univ. Sci. B 7, 299 (2006). doi 10.1631/jzus.2006.B0299
S. S. Rayalu, D. Jose, P. A. Mangrulkar, et al., Int. J. Hydrogen Energy 39, 3617 (2014). doi 10.1016/j.ijhydene.2013.11.120
N. J. G. Xue Ming Wang, Guang Jun Wu, and Lan Dong Li, Adv. Mater. Res. 148, 1258 (2010).
M. Haruta, Catal. Today 36, 153 (1997) doi 10.1016/S0920-5861(96)00208-8
S. S. Rayalu, D. Jose, M. V. Joshi, et al., Appl. Catal. B: Environ. 142, 684 (2013). doi 10.1016/j.apcatb.2013.05.057
J. Taing, M. H. Cheng, and J. C. Hemminger, ACS Nano 5, 6325 (2011). doi 10.1021/nn201396v
Y. Hu, X. Song, S. Jiang, and C. Wei, Chem. Eng. J. 274, 102 (2015). doi 10.1016/j.cej.2015.03.135
M. Maicu, M. C. Hidalgo, G. Colon, and J. A. Navio, J. Photochem. Photobiol. A: Chem. 217, 275 (2011). doi 10.1016/j.jphotochem.2010.10.020
S. C. Chan and M. A. Barteau, Langmuir 21, 5588 (2005). doi 10.1021/la046887k
L. Wen, B. Liu, C. Liu, and X. Zhao, J. Wuhan Univ. Technol. Mater. Sci. Ed. 24, 258 (2009). doi 10.1007/sl1595-009-2258-210.1007/sl
H. Wang, T. You, W. Shi, et al., J. Phys. Chem. C 116, 6490 (2012). doi 10.1021/jp212303q
A. Tanaka, S. Sakaguchi, K. Hashimoto, and H. Kominami, ACS Catal. 3, 79 (2013). doi 10.1021/cs3006499
O. Nicoletti, F. de la Pena, R. K. Leary,et al., Nature 502, 80 (2013).
S. A. Maier and H. A. Atwater, J. Appl. Phys. 98, 11101 (2005). doi 10.1063/1.1951057
C. Gomes Silva, R. Juarez, T. Marino, et al., J. Am. Chem. Soc. 133, 595 (2011). doi 10.1021/jal086358
R. Rhodes, M. Beliatis, C. Smith, et al., ATI Univ. Surrey 238, 5358 (1972).
R. M. Navarro Yerga, M. C. Alvarez Galvan, F. del Valle, et al., ChemSusChem. 2, 471 (2009). doi 10.1002/cssc.200900018
V. Iliev, D. Tomova, L. Bilyarska, and G. Tyuliev, J.Mol. Catal. A: Chem 263, 32 (2007). doi 10.1016/j.molcata.2006.08.019
M. Murdoch, G. I. N. Watergiyse, M. A. Nadeem, et al., Nat. Chem. 3, 489 (2011).
E. Kowalska, S. Rau, and B. Ohtani, J. Nanotechnol. 11 (2012). doi 10.1155/2012/361853
X. Huang and M. A. El-Sayed, J. Adv. Res. 1, 13 (2010). doi 10.1016/j.jare.2010.02.002
P. R. Chandran, M. Naseer, N. Udupa, and N. Sandhyarani, Nanotechnology 23, 15602 (2011). doi 10.1088/0957-4484/23/1/015602
S. Kumar, K. S. Gandhi, and R. Kumar, Ind. Eng. Chem. Res. 46, 3128 (2007). doi 10.1021/ie060672j
O. Neumann, A. S. Urban, J. Day, et al., ACS Nano 7, 42 (2013). doi 10.1021/nn304948h
A. Polman, ACS Nano 7, 15 (2013). doi 10.1021/nn305869y
D. Han, Z. Meng, D. Wu, et al., Nanoscale Res. Lett. 6, 457 (2011). doi 10.1186/1556-276X-6-457
R. Kydd, J. Scott, W. Y. Teoh, et al., Langmuir 26, 2099 (2010). doi 10.1021/la902592p
M. Harada, K. Okamoto, and M. Terazima, Langmuir 22, 9142 (2006). doi 10.1021/la061663i
P. E. Hopkins, J. C. Duda, R. N. Salaway, et al., Nanoscale Microscale Thermophys. Eng. 12, 320 (2008). doi 10.1080/15567260802591985
M. S. Dresselhaus, Solid State Physics, Part II: Optical Properties of Solids (2001).
A. Bumajdad and M. Madkour, Phys. Chem. Chem. Phys. 16, 7146 (2014). doi 10.1039/c3cp54411g
D. Tsukamoto, Y. Shiraishi, Y. Sugano, et al., J. Am. Chem. Soc. 134, 6309 (2012). doi 10.1021/ja2120647
A. Primo, A. Corma, and H. Garcia, Phys. Chem. Chem. Phys. 13, 886 (2011). doi 10.1039/C0CP00917B
Z. W. Seh, S. Liu, M. Low, et al., Adv. Mater. 24, 2310 (2012). doi 10.1002/adma.201104241
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Hippargi, G., Maddigapu, P.R., Labhsetwar, N. et al. Titania Gold Composite: Effect of Illumination on Size of Gold Nanoparticles with Consequent Implication on Photocatalytic Water Splitting. Russ. J. Phys. Chem. B 11, 1002–1011 (2017). https://doi.org/10.1134/S1990793117060215
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793117060215