Abstract
Purpose
In approximately 30% of triple-negative breast cancer (TNBC) patients a complete pathological response is achieved. However, after neo-adjuvant chemotherapy treatment (NACT) residual tumour cells can be intrinsically resistant to chemotherapy. In this study, associations of the WNT/beta-catenin pathway with chemo-tolerance of NACT treated TNBC patients were compared to that of pre-treatment TNBC patients.
Methods
Expression analyses were performed in both pre-treatment and NACT treated TNBC samples using immunohistochemistry and qRT-PCR, along with DNA copy number variation (CNV) and promoter methylation analyses to elucidate the mechanism(s) underlying chemo-tolerance. In addition, in vitro validation experiments were performed in TNBC cells followed by in vivo clinicopathological correlation analyses.
Results
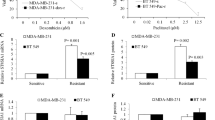
A reduced expression (41.1%) of nuclear beta-catenin together with a low proliferation index was observed in NACT samples, whereas a high expression (59.0%) was observed in pre-treatment samples. The reduced nuclear expression of beta-catenin in the NACT samples showed concordance with reduced expression levels (47-52.9%) of its associated receptors (FZD7 and LRP6) and increased expression levels (35.2–41.1%) of its antagonists (SFRP1, SFRP2, DKK1) compared to those in the pre-treatment samples. The expression levels of the receptors showed no concordance with its respective gene copy number/mRNA expression statuses, regardless treatment. Interestingly, however, significant increases in promoter hypomethylation of the antagonists were observed in the NACT samples compared to the pre-treatment samples. Similar expression patterns of the antagonists, receptors and beta-catenin were observed in the TNBC-derived cell line MDA-MB-231 using the anthracyclines doxorubicin and nogalamycin, suggesting the importance of promoter hypomethylation in chemotolerance. NACT patients showing reduced receptor and/or beta-catenin expression levels and high antagonist expression levels exhibited a comparatively better prognosis than the pre-treatment patients.
Conclusions
Our data suggest that reduced nuclear expression of beta-catenin in NACT TNBC samples, due to downregulation of its receptors and upregulation of its antagonists through promoter hypomethylation of the WNT pathway, plays an important role in chemo-tolerance.







Similar content being viewed by others
Abbreviations
- PT:
-
Pre-treatment
- NT:
-
NACT
- 5-aza-dC:
-
5-Aza-2′-deoxycytidine
References
W.D. Foulkes, I.E. Smith, J.S. Reis-Filho, Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010)
E.K. Millar, P.H. Graham, S.A. O’Toole, C.M. McNeil, L. Browne, A.L. Morey, S. Eggleton, J. Beretov, C. Theocharous, A. Capp, E. Nasser, J.H. Kearsley, G. Delaney, G. Papadatos, C. Fox, R.L. Sutherland, Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J. Clin. Oncol. 27, 4701–4708 (2009)
K.D. Voduc, M.C. Cheang, S. Tyldesley, K. Gelmon, T.O. Nielsen, H. Kennecke, Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 28, 1684–1691 (2010)
P. Samadi, S. Saki, F.K. Dermani, M. Pourjafar, M. Saidijam, Emerging ways to treat breast cancer: will promises be met? Cell. Oncol. 41, 605–621 (2018)
M.L. Pecero, J. Salvador-Bofill, S. Molina-Pinelo, Long non-coding RNAs as monitoring tools and therapeutic targets in breast cancer. Cell. Oncol. 42, 1–12 (2018)
T. Foukakis, G. von Minckwitz, N.O. Bengtsson, Y. Brandberg, B. Wallberg, T. Fornander, B. Mlineritsch, S. Schmatloch, C.F. Singer, G. Steger, D. Egle, E. Karlsson, L. Carlsson, S. Loibl, M. Untch, M. Hellstrom, H. Johansson, H. Anderson, P. Malmstrom, M. Gnant, R. Greil, V. Mobus, J. Bergh, Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival among women with high-risk early breast cancer: a randomized clinical trial. Jama 316, 1888–1896 (2016)
G.S. Sandhu, S. Erqou, H. Patterson, A. Mathew, Prevalence of triple-negative breast cancer in India: Systematic review and meta-analysis. J. Glob. Oncol. 2, 412–421 (2016)
H. Charfare, S. Limongelli, A.D. Purushotham, Neoadjuvant chemotherapy in breast cancer. Br. J. Surg. 92, 14–23 (2005)
B. Fisher, J. Bryant, N. Wolmark, E. Mamounas, A. Brown, E.R. Fisher, D.L. Wickerham, M. Begovic, A. DeCillis, A. Robidoux, R.G. Margolese, A.B. Cruz Jr., J.L. Hoehn, A.W. Lees, N.V. Dimitrov, H.D. Bear, Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 16, 2672–2685 (1998)
N. Wolmark, J. Wang, E. Mamounas, J. Bryant, B. Fisher, Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. 96–102 (2001)
H.M. Kuerer, A.A. Sahin, K.K. Hunt, L.A. Newman, T.M. Breslin, F.C. Ames, M.I. Ross, A.U. Buzdar, G.N. Hortobagyi, S.E. Singletary, Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann. Surg. 230, 72–78 (1999)
V. Guarneri, K. Broglio, S.W. Kau, M. Cristofanilli, A.U. Buzdar, V. Valero, T. Buchholz, F. Meric, L. Middleton, G.N. Hortobagyi, and A.M. Gonzalez-Angulo, Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J. Clin. Oncol. 24, 1037–1044 (2006)
C. Liedtke, C. Mazouni, K.R. Hess, F. Andre, A. Tordai, J.A. Mejia, W.F. Symmans, A.M. Gonzalez-Angulo, B. Hennessy, M. Green, M. Cristofanilli, G.N. Hortobagyi, L. Pusztai, Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 26, 1275–1281 (2008)
H. Dasgupta, N. Mukherjee, S. Islam, R. Bhattacharya, N. Alam, A. Roy, S. Roychoudhury, J. Biswas, C.K. Panda, Frequent alterations of homologous recombination repair pathway in primary and chemotolerant breast carcinomas: clinical importance. Future Oncol. (London, England) 13, 159–174 (2016)
P.M. Spanheimer, R.W. Askeland, M.V. Kulak, T. Wu, R.J. Weigel, High TFAP2C/low CD44 expression is associated with an increased rate of pathologic complete response following neoadjuvant chemotherapy in breast cancer. J Surg Res 184, 519–525 (2013)
R.X. Wang, S. Chen, X. Jin, Z.M. Shao, Value of Ki-67 expression in triple-negative breast cancer before and after neoadjuvant chemotherapy with weekly paclitaxel plus carboplatin. Sci Rep. 6, 30091 (2016)
D.G. Stover, J.L. Coloff, W.T. Barry, J.S. Brugge, E.P. Winer, L.M. Selfors, The role of proliferation in determining response to neoadjuvant chemotherapy in breast cancer: a gene expression-based meta-analysis. Clin. Cancer Res. 22, 6039–6050 (2016)
C.M. Yang, S. Ji, Y. Li, L.Y. Fu, T. Jiang, F.D. Meng, β-Catenin promotes cell proliferation, migration, and invasion but induces apoptosis in renal cell carcinoma. Onco Targets Ther. 10, 711–724 (2017)
D.B. Shieh, R.Y. Li, J.M. Liao, G.D. Chen, Y.M. Liou, Effects of genistein on beta-catenin signaling and subcellular distribution of actin-binding proteins in human umbilical CD105-positive stromal cells. J. Cell. Physiol. 223, 423–434 (2010)
T. Gruosso, V. Mieulet, M. Cardon, B. Bourachot, Y. Kieffer, F. Devun, T. Dubois, M. Dutreix, A. Vincent-Salomon, K.M. Miller, F. Mechta-Grigoriou, Chronic oxidative stress promotes H2AX protein degradation and enhances chemosensitivity in breast cancer patients. EMBO Mol Med. 8, 527–549 (2016)
E. Stickeler, D. Pils, M. Klar, M. Orlowsk-Volk, A. Zur Hausen, M. Jager, D. Watermann, G. Gitsch, R. Zeillinger, C.B. Tempfer, Basal-like molecular subtype and HER4 up-regulation and response to neoadjuvant chemotherapy in breast cancer. Oncol. Rep. 26, 1037–1045 (2011)
L.A. Korde, L. Lusa, L. McShane, P.F. Lebowitz, L. Lukes, K. Camphausen, J.S. Parker, S.M. Swain, K. Hunter, J.A. Zujewski, Gene expression pathway analysis to predict response to neoadjuvant docetaxel and capecitabine for breast cancer. Breast Cancer Res. Treat. 119, 685–699 (2010)
R.K.S. Dewi, S. Pramana, S.I. Wanandi, Journal of Physics: Conference Series, (2018)
M. Rosa, H.S. Han, R. Ismail-Khan, P. Allam-Nandyala, M.M. Bui, Beta-catenin expression patterns in matched pre- and post-neoadjuvant chemotherapyresistant breast cancer. Ann. Clin. Lab. Sci. 45, 10–16 (2015)
L. Yang, X. Wu, Y. Wang, K. Zhang, J. Wu, Y.C. Yuan, X. Deng, L. Chen, C.C. Kim, S. Lau, G. Somlo, Y. Yen, FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 30, 4437–4446 (2011)
N. Dey, B. Young, M. Abramovitz, M. Bouzyk, B. Barwick, P. De, B. Leyland-Jones, Differential activation of Wnt-β-catenin pathway in triple negative breast cancer increases MMP7 in a PTEN dependent manner. PloS One 8:e77425 (2016)
J. Ma, W. Lu, D. Chen, B. Xu, Y. Li, Role of Wnt co-receptor LRP6 in triple negative breast cancer cell migration and invasion. J. Cell. Biochem. 118, 2968–2976 (2017)
N. Mukherjee, N. Bhattacharya, N. Alam, A. Roy, S. Roychoudhury, C.K. Panda, Subtype-specific alterations of the Wnt signaling pathway in breast cancer: clinical and prognostic significance. Cancer Sci. 103, 210–220 (2012)
Y.J. Jeong, H.Y. Jeong, J.G. Bong, S.H. Park, H.K. Oh, Low methylation levels of the SFRP1 gene are associated with the basal-like subtype of breast cancer. Oncol. Rep. 29, 1946–1954 (2013)
C. Bernemann, C. Hulsewig, C. Ruckert, S. Schafer, L. Blumel, G. Hempel, M. Gotte, B. Greve, P.J. Barth, L. Kiesel, C. Liedtke, Influence of secreted frizzled receptor protein 1 (SFRP1) on neoadjuvant chemotherapy in triple negative breast cancer does not rely on WNT signaling. Mol. Cancer 13, 174 (2014)
J. Wang, M. Li, D. Chen, J. Nie, Y. Xi, X. Yang, Y. Chen, Z. Yang, Expression of C-myc and β-catenin and their correlation in triple negative breast cancer. Minerva Med. 108, 513–517 (2017)
W.H. Xu, Z.B. Liu, C. Yang, W. Qin, Z.M. Shao, Expression of dickkopf-1 and beta-catenin related to the prognosis of breast cancer patients with triple negative phenotype. PloS One 7, e37624 (2012)
G.P. Maiti, P. Mondal, N. Mukherjee, A. Ghosh, S. Ghosh, S. Dey, J. Chakrabarty, A. Roy, J. Biswas, S. Roychoudhury, C.K. Panda, Overexpression of EGFR in head and neck squamous cell carcinoma is associated with inactivation of SH3GL2 and CDC25A genes. PloS One 8, e63440 (2013)
J. Sambrook, D.W. Russell, J. Sambrook, The condensed protocols from Molecular cloning: a laboratory manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., 2006)
F. Perrone, S. Suardi, E. Pastore, P. Casieri, M. Orsenigo, S. Caramuta, G. Dagrada, M. Losa, L. Licitra, P. Bossi, S. Staurengo, M. Oggionni, L. Locati, G. Cantu, M. Squadrelli, A. Carbone, M.A. Pierotti, S. Pilotti, Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin. Cancer Res. 12, 6643–6651 (2006)
P. Chomczynski, N. Sacchi, Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987)
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 (2001)
T.D. Schmittgen, K.J. Livak, Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008)
N. Bhattacharya, A. Roy, B. Roy, S. Roychoudhury, C.K. Panda, MYC gene amplification reveals clinical association with head and neck squamous cell carcinoma in Indian patients. J. Oral Pathol. Med. 38, 759–763 (2009)
K. Li, H. Du, X. Lian, S. Yang, D. Chai, C. Wang, R. Yang, X. Chen, Characterization of β2-microglobulin expression in different types of breast cancer. BMC Cancer 14, 750 (2014)
S.H. Park, Y.M. Chung, J. Ma, Q. Yang, J.S. Berek, M.C. Hu, Pharmacological activation of FOXO3 suppresses triple-negative breast cancer in vitro and in vivo. Oncotarget 7, 42110–42125 (2016)
J. Lopez-Rios, P. Esteve, J.M. Ruiz, P. Bovolenta, The Netrin-related domain of Sfrp1 interacts with Wnt ligands and antagonizes their activity in the anterior neural plate. Neu. Dev. 3, 19 (2008)
A. Tripathi, S. Banerjee, A. Roy, S. Roychowdhury, C.K. Panda, Alterations of the P16 gene in uterine cervical carcinoma from Indian patients. Int. J. Gynecol. Cancer 13, 472–479 (2003)
V.I. Loginov, A.V. Maliukova, A. Seregin Iu, D.S. Khodyrev, T.P. Kazubskaia, V.D. Ermilova, R.F. Gar’kavtseva, L.L. Kiselev, E.R. Zabarovskii, E.A. Braga, Methylation of the promoter region of the RASSF1A gene, a candidate tumor suppressor, in primary epithelial tumors. Mol. Biol. (Mosk) 38, 654–667 (2004)
H. Thomassin, C. Kress, T. Grange, MethylQuant: a sensitive method for quantifying methylation of specific cytosines within the genome. Nucleic Acids Res. 32, e168 (2004)
H. Dasgupta, S. Islam, N. Alam, A. Roy, S. Roychoudhury, C.K. Panda, Hypomethylation of mismatch repair genes MLH1 and MSH2 is associated with chemotolerance of breast carcinoma: Clinical significance. J. Surg. Oncol. 119, 88–100 (2018)
S. Dasgupta, N. Mukherjee, S. Roy, A. Roy, A. Sengupta, S. Roychowdhury, C.K. Panda, Mapping of the candidate tumor suppressor genes’ loci on human chromosome 3 in head and neck squamous cell carcinoma of an Indian patient population. Oral Oncol. 38, 6–15 (2002)
N. Mukherjee, H. Dasgupta, R. Bhattacharya, D. Pal, R. Roy, S. Islam, N. Alam, J. Biswas, A. Roy, S. Roychoudhury, C.K. Panda, Frequent inactivation of MCC/CTNNBIP1 and overexpression of phospho-beta-catenin(Y654) are associated with breast carcinoma: clinical and prognostic significance. Biochim. Biophys. Acta 1862, 1472–1484 (2016)
H. Dasgupta, M.S. Islam, N. Alam, A. Roy, S. Roychoudhury, C.K. Panda, I nduction of HRR genes and inhibition of DNMT1 is associated with anthracycline anti-tumor antibiotic-tolerant breast carcinoma cells. Mol. Cell. Biochem. 453, 163–178 (2019)
D. Mazumder (Indra), S. Mitra, R.K. Singh, S. Dutta, A. Roy, R.K. Mondal, P.S. Basu, S. Roychoudhury, C.K. Panda, Inactivation of CHEK1 and EI24 is associated with the development of invasive cervical carcinoma: clinical and prognostic implications. Int. J. Cancer 129, 1859–1871 (2011)
D. Olmeda, S. Castel, S. Vilaro, A. Cano, Beta-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol. Biol. Cell 14, 2844–2860 (2003)
S. Pradhan, J.L. Sperduto, C.J. Farino, J.H. Slater, Engineered in vitro models of tumor dormancy and reactivation. J. Biol. Eng. 12, 37 (2018)
F. Rossari, C. Zucchinetti, G. Buda, E. Orciuolo, Tumor dormancy as an alternative step in the development of chemoresistance and metastasis - clinical implications. Cell. Oncol. 43, 155–176 (2020)
R. Han, J. Xiong, R. Xiao, E. Altaf, J. Wang, Y. Liu, H. Xu, Q. Ding, Q. Zhang, Activation of β-catenin signaling is critical for doxorubicin-induced epithelial-mesenchymal transition in BGC-823 gastric cancer cell line. Tumour Biol. 34, 277–284 (2013)
H. Sakane, H. Yamamoto, A. Kikuchi, LRP6 is internalized by Dkk1 to suppress its phosphorylation in the lipid raft and is recycled for reuse. J. Cell Sci. 123, 360–368 (2010)
H. Yamamoto, H. Sakane, H. Yamamoto, T. Michiue, A. Kikuchi, Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev. Cell 15, 37–48 (2008)
C.C. Liu, T. Kanekiyo, B. Roth, G. Bu, Tyrosine-based signal mediates LRP6 receptor endocytosis and desensitization of Wnt/β-catenin pathway signaling. J. Biol. Chem. 289, 27562–27570 (2014)
C.H. Teo, T. Soga, I.S. Parhar, Brain Beta-Catenin Signalling During Stress and Depression. Neurosignals 26, 31–42 (2018)
E. Tiligada, Chemotherapy: induction of stress responses. Endocr. Relat. Cancer 13(Suppl 1), 115–124 (2006)
W. Fiskus, K. Buckley, R. Rao, A. Mandawat, Y. Yang, R. Joshi, Y. Wang, R. Balusu, J. Chen, S. Koul, A. Joshi, S. Upadhyay, P. Atadja, K.N. Bhalla, Panobinostat treatment depletes EZH2 and DNMT1 levels and enhances decitabine mediated de-repression of JunB and loss of survival of human acute leukemia cells. Cancer Biol. Ther. 8, 939–950 (2009)
N.U. Lin, A. Vanderplas, M.E. Hughes, R.L. Theriault, S.B. Edge, Y.N. Wong, D.W. Blayney, J.C. Niland, E.P. Winer, J.C. Weeks, Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118, 5463–5472 (2012)
Acknowledgements
The authors thank the Director of the Chittaranjan National Cancer Institute, Kolkata, India. They also thank Dr. Partha Sarathi Dasgupta, Emeritus Scientist, Chittaranjan National Cancer Institute, for his valuable suggestions during the study. We would like to thank Mr. Anirban Roychowdhury and Dr. Sudip Samadder for their technical assistance. Financial support for this work was provided by UGC-NET Fellowship grant F.2–3/2000 (SA-I) (Sr. No. 2061030813, Ref. No.: 20 − 06/2010(i) EU-IV dated 22.10.2010) to Mrs. H. Dasgupta and to Mr. Arijit Das for his editing and proofreading support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no actual or potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Strategy of utilization of the primary TNBC samples in different experimental analyses. N: number of samples; IHC: immunohistochemistry. (PNG 279 kb)
Fig. S2
Representative immunoblots of (a) alpha-tubulin for the cytoplasmic specific marker and (b) histone 3 (H3) for the nuclear specific marker. (PNG 235 kb)
Table S1
(DOC 42 kb)
Table S2
(DOC 87 kb)
Table S3
(DOC 49 kb)
Table S4
(DOC 99 kb)
Table S5
(DOC 53 kb)
Table S6
(DOC 41 kb)
Table S7
(DOC 60 kb)
Table S8
(DOC 63 kb)
Rights and permissions
About this article
Cite this article
Islam, S., Dasgupta, H., Basu, M. et al. Downregulation of beta-catenin in chemo-tolerant TNBC through changes in receptor and antagonist profiles of the WNT pathway: Clinical and prognostic implications. Cell Oncol. 43, 725–741 (2020). https://doi.org/10.1007/s13402-020-00525-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00525-5