Abstract
Purpose
Although it has been reported that up-regulation of phosphofructokinase (PFK) expression is a major feature of malignant tumors, the role of platelet type PFK (PFKP) in tumor initiation and progression is as yet poorly understood. The objective of this study was to evaluate PFKP expression in lung cancer and its effect on glycolysis, and to explore correlations between PFKP expression levels and clinical lung cancer patient features.
Methods
PFKP mRNA expression levels in cancer tissues and adjacent normal tissues were compared using The Cancer Genome Atlas (TCGA) database. PFKP mRNA and protein expression levels in fresh lung cancer tissues and cell lines were assessed using quantitative real-time PCR and Western blotting. Immunohistochemistry (IHC) was used to assess PFKP expression in 150 archival lung adenocarcinoma samples, after which follow-up data and their correlations with clinical features and patient prognosis were evaluated. A retroviral shRNA-mediated method was used to construct stable cell lines expressing low levels of PFKP. Glucose, lactate and adenosine triphosphate concentrations in the cell culture supernatants were determined using enzymatic, spectrophotometric and enzyme-linked immunosorbent (ELISA) assays, respectively. The effect of PFKP expression on the proliferation of lung cancer cells was assessed using colony forming, MTT and flow cytometry assays, respectively. Finally, data from tissue samples of 533 patients with lung adenocarcinoma and 502 patients with lung squamous cell carcinoma were downloaded from the TCGA database, after which pathway and gene correlation information was retrieved using gene set enrichment analyses.
Results
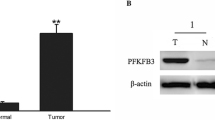
We found that PFKP was highly expressed in lung cancer tissues and cell lines. Using IHC we found that PFKP was highly expressed in primary lung adenocarcinoma tissues and that a high expression was associated with a poor prognosis. Clinical data analysis revealed that the PFKP expression levels correlated with tumor size and patient survival. Lung cancer cell lines with decreased PFKP expression levels showed significant decreases in glucose uptake rates, lactate levels and adenosine triphosphate concentrations. They also exhibited significantly decreased proliferation rates, colony forming abilities and increased G2/M cell cycle phase percentages. Gene set enrichment analysis revealed that multiple pathways, including glycolytic pathways, may be involved in the regulation PFKP.
Conclusions
Our data indicate that PFKP is highly expressed in lung cancer tissues and cell lines and is associated with tumor size and patient prognosis. As such, PFKP may serve as a prognostic biomarker. We also found that PFKP regulates the level of glycolysis in lung cancer cells and is associated with lung cancer cell proliferation. These data may be instrumental for the design of new lung cancer treatment options.









Similar content being viewed by others
Abbreviations
- IHC:
-
Immunohistochemistry
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- FBS:
-
Fetal Bovine Serum;
- qRT-PCR:
-
Quantitative Reverse Transcriptase PCR
- NSCLC:
-
Non-Small Cell Lung Carcinoma
- PFK-1:
-
Phosphofructokinase-1
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- TCGA:
-
The Cancer Genome Atlas
- GSEA:
-
Gene Set Enrichment Analysis
References
R. Siegel, D. Naishadham, A. Jemal, Cancer statistics, 2013. CA Cancer J Clin 63, 11–30 (2013)
C.M. North, D.C. Christiani, Women and lung cancer: What is new? Semin Thorac Cardiovasc Surg 25, 87–94 (2013)
P.P. Hsu, D.M. Sabatini, Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 (2008)
R.A. Cairns, I.S. Harris, T.W. Mak, Regulation of cancer cell metabolism. Nat Rev Cancer 11, 85–95 (2011)
G. van Niekerk, A.M. Engelbrecht, Role of PKM2 in directing the metabolic fate of glucose in cancer: A potential therapeutic target. Cell Oncol 41, 343–351 (2018)
I. Mor, E.C. Cheung, K.H. Vousden, Control of glycolysis through regulation of PFK1: Old friends and recent additions. Cold Spring Harb Symp Quant Biol 76, 211–216 (2011)
R. Moreno-Sanchez, E. Saavedra, Energy metabolism in tumor cells. FEBS J 274, 1393–1418 (2007)
P. Nilendu, S.C. Sarode, D. Jahagirdar, I. Tandon, S. Patil, G.S. Sarode, J.K. Pal, N.K. Sharma, Mutual concessions and compromises between stromal cells and cancer cells: Driving tumor development and drug resistance. Cell Oncol 41, 353–367 (2018)
C. Sanchez-Martnez, J.J. Aragon, Analysis of phosphofructokinase subunits and isozymes in ascites tumor cells and its original tissue, murine mammary gland. FEBS Lett 409, 86–90 (1997)
G. Wang, Z. Xu, C. Wang, F. Yao, J. Li, C. Chen, S. Sun, Differential phosphofructokinase-1 isoenzyme patterns associated with glycolytic efficiency in human breast cancer and paracancer tissues. Oncol Lett 6, 1701–1706 (2013)
R.J. Shaw, Glucose metabolism and cancer. Curr Opin Cell Biol 18, 598–608 (2006)
R.A. Gatenby, R.J. Gillies, Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4, 891–899 (2004)
K. Zhou, Y.L. Yao, Z.C. He, C. Chen, X.N. Zhang, K.D. Yang, Y.Q. Liu, Q. Liu, W.J. Fu, Y.P. Chen, Q. Niu, Q.H. Ma, R. Zhou, X.H. Yao, X. Zhang, Y.H. Cui, X.W. Bian, Y. Shi, Y.F. Ping, VDAC2 interacts with PFKP to regulate glucose metabolism and phenotypic reprogramming of glioma stem cells. Cell Death Dis 9, 988 (2018)
L. Lang, R. Chemmalakuzhy, C. Shay, Y. Teng, PFKP signaling at a glance: An emerging mediator of Cancer cell metabolism. Adv Exp Med Biol 1134, 243–258 (2019)
Y. Wang, Q. Mei, Y.Q. Ai, R.Q. Li, L. Chang, Y.F. Li, Y.X. Xia, W.H. Li, Y. Chen, Identification of lung cancer oncogenes based on the mRNA expression and single nucleotide polymorphism profile data. Neoplasma 62, 966–973 (2015)
J.H. Lee, R. Liu, J. Li, C. Zhang, Y. Wang, Q. Cai, X. Qian, Y. Xia, Y. Zheng, Y. Piao, Q. Chen, J.F. de Groot, T. Jiang, Z. Lu, Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun 8, 949 (2017)
J.H. Lee, R. Liu, J. Li, Y. Wang, L. Tan, X.J. Li, X. Qian, C. Zhang, Y. Xia, D. Xu, W. Guo, Z. Ding, L. Du, Y. Zheng, Q. Chen, P.L. Lorenzi, G.B. Mills, T. Jiang, Z. Lu, EGFR-phosphorylated platelet isoform of phosphofructokinase 1 promotes PI3K activation. Mol Cell 70, 197–210 (2018)
J. Wang, P. Zhang, J. Zhong, M. Tan, J. Ge, L. Tao, Y. Li, Y. Zhu, L. Wu, J. Qiu, X. Tong, The platelet isoform of phosphofructokinase contributes to metabolic reprogramming and maintains cell proliferation in clear cell renal cell carcinoma. Oncotarget 7, 27142–27157 (2016)
C.V. Dang, Links between metabolism and cancer. Genes Dev 26, 877–890 (2012)
G. Kroemer, J. Pouyssegur, Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 13, 472–482 (2008)
C.V. Dang, M. Hamaker, P. Sun, A. Le, P. Gao, Therapeutic targeting of cancer cell metabolism. J Mol Med 89, 205–212 (2011)
G. Chen, H. Liu, Y. Zhang, J. Liang, Y. Zhu, M. Zhang, D. Yu, C. Wang, J. Hou, Silencing PFKP inhibits starvation-induced autophagy, glycolysis, and epithelial mesenchymal transition in oral squamous cell carcinoma. Exp Cell Res 370, 46–57 (2018)
J.D. Gordan, C.B. Thompson, M.C. Simon, HIF and c-Myc: Sibling rivals for control of Cancer cell metabolism and proliferation. Cancer Cell 12, 108–113 (2007)
A. Ramanathan, C. Wang, S.L. Schreiber, Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci U S A 102, 5992–5997 (2005)
S. Ganapathy-Kanniappan, Linking tumor glycolysis and immune evasion in cancer: Emerging concepts and therapeutic opportunities. Biochim Biophys Acta Rev Cancer 1868, 212–220 (2017)
L. Baitsch, P. Baumgaertner, E. Devêvre, S.K. Raghav, A. Legat, L. Barba, S. Wieckowski, H. Bouzourene, B. Deplancke, P. Romero, N. Rufer, D.E. Speiser, Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest 121, 2350–2360 (2011)
M. Peng, D. Yang, Y. Hou, S. Liu, M. Zhao, Y. Qin, R. Chen, Y. Teng, M. Liu, Intracellular citrate accumulation by oxidized ATM-mediated metabolism reprogramming via PFKP and CS enhances hypoxic breast cancer cell invasion and metastasis. Cell Death Dis 10, 228 (2019)
M.T. Bjerre, S.H. Strand, M. Nørgaard, H. Kristensen, A.K. Rasmussen, M.M. Mortensen, J. Fredsøe, P. Mouritzen, B. Ulhøi, T. Ørntoft, M. Borre, K.D. Sørensen, Aberrant DOCK2, GRASP, HIF3A and PKFP hypermethylation has potential as a prognostic biomarker for prostate cancer. Int J Mol Sci 20, pii: E1173 (2019)
Y. He, F. Deng, S. Zhao, S. Zhong, J. Zhao, D. Wang, X. Chen, J. Zhang, J. Hou, W. Zhang, L. Ding, J. Tang, Z. Zhou, Analysis of miRNA-mRNA network reveals miR-140-5p as a suppressor of breast cancer glycolysis via targeting GLUT1. Epigenomics 11, 1021–1036 (2019)
S.Y. Lee, C.C. Jin, J.E. Choi, M.J. Hong, D.K. Jung, S.K. Do, S.A. Baek, H.J. Kang, H.G. Kang, S.H. Choi, W.K. Lee, Y. Seok, E.B. Lee, J.Y. Jeong, K.M. Shin, S. Cho, S.S. Yoo, J. Lee, S.I. Cha, C.H. Kim, Y.M. Lee, I.K. Lee, S. Jheon, J.Y. Park, Genetic polymorphisms in glycolytic pathway are associated with the prognosis of patients with early stage non-small cell lung cancer. Sci Rep 6, 35603 (2016)
Acknowledgements
The authors thank Taizhou Hospital of Zhejiang Province for providing the research environment.
Availability of data and materials
All data generated or analyzed in this study are included in this published article.
Funding
This study was funded by Zhejiang Provincial Natural Fund (grant number LQ18H160029 and Y19H160116).
Author information
Authors and Affiliations
Contributions
JF, ZJ, & HL searched the literature and drafted and revised the manuscript. KJ & KBJ processed the data. CZ and BC revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration, which was approved by the Medical Ethics Committee of Taizhou Hospital, Zhejiang Province, China. Written informed consent to participate in the study was obtained from all individual participants or their guardians.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 26.0 kb)
Rights and permissions
About this article
Cite this article
Shen, J., Jin, Z., Lv, H. et al. PFKP is highly expressed in lung cancer and regulates glucose metabolism. Cell Oncol. 43, 617–629 (2020). https://doi.org/10.1007/s13402-020-00508-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00508-6