Abstract
Purpose
Given its extremely poor prognosis, there is a pressing need for an improved understanding of the biology of glioblastoma multiforme (GBM), including the roles of tumor subpopulations that may contribute to their growth rate and therapy resistance. The most malignant phenotypes of GBM have been ascribed to the presence of subpopulations of cancer stem cells (CSCs), which are resistant to chemotherapeutic drugs and ionizing radiation and which promote invasiveness and metastasis. The mechanisms by which the CSC state is obtained and by which it promotes tumor maintenance are only beginning to emerge. We hypothesize that M2 polarized macrophages may affect CSC phenotypes via cell-cell communication.
Methods
We investigated the interplay between glioma CSCs and macrophages via co-culture. The invasiveness of CSCs in the absence and presence of macrophages was assessed using collagen degradation and Transwell migration assays. The role of STAT3 as a CSC phenotypic mediator was assessed using siRNA-mediated gene silencing.
Results
We found that the levels of a M2 macrophage-specific secreted cytokine, TGF-β1, were elevated in the presence of CSCs, regardless of whether the cells were plated as contacting or non-contacting co-cultures. In addition, we found that the co-culture resulted in enhanced expression of M2 markers in macrophages that were previously polarized to the M1 phenotype. siRNA-mediated STAT3 silencing was found to reduce the chemo-responsiveness and migratory abilities of the CSCs. Combination treatment of STAT3 siRNA and DNA alkylating agents was found to further abrogate CSC functions.
Conclusions
Our data indicate that the co-culture of CSCs and macrophages results in bi-directional signaling that alters the phenotypes of both cell types. These results provide an explanation for recently observed effects of macrophages on GBM tumor cell growth, motility and therapeutic resistance, and suggest potential therapeutic strategies to disrupt the CSC phenotype by impairing its communication with macrophages.







Similar content being viewed by others
References
Z. Li, J.W. Lee, D. Mukherjee, J. Ji, S.P. Jeswani, K.L. Black, J.S. Yu, Immunotherapy targeting glioma stem cells--insights and perspectives. Expert. Opin. Biol. Ther. 12, 165–178 (2012)
M. Staberg, S.R. Michaelsen, R.D. Rasmussen, M. Villingshoj, H.S. Poulsen, P. Hamerlik, Inhibition of histone deacetylases sensitizes glioblastoma cells to lomustine. Cell. Oncol. 40, 21–32 (2017)
D. Matias, J. Balca-Silva, L.G. Dubois, B. Pontes, V.P. Ferrer, L. Rosario, A. do Carmo, J. Echevarria-Lima, A.B. Sarmento-Ribeiro, M.C. Lopes, V. Moura-Neto, Dual treatment with shikonin and temozolomide reduces glioblastoma tumor growth, migration and glial-to-mesenchymal transition. Cell. Oncol. 40, 247–261 (2017)
H.D. Hemmati, I. Nakano, J.A. Lazareff, M. Masterman-Smith, D.H. Geschwind, M. Bronner-Fraser, H.I. Kornblum, Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. U. S. A. 100, 15178–15183 (2003)
B. Ortensi, M. Setti, D. Osti, G. Pelicci, Cancer stem cell contribution to glioblastoma invasiveness. Stem Cell Res. Ther. 4, 18 (2013)
A. Chavez-Gonzalez, B. Bakhshinejad, K. Pakravan, M.L. Guzman, S. Babashah, Novel strategies for targeting leukemia stem cells: Sounding the death knell for blood cancer. Cell. Oncol. (Dordr.) 40, 1–20 (2017)
J.M. Heddleston, M. Hitomi, M. Venere, W.A. Flavahan, K. Yang, Y. Kim, S. Minhas, J.N. Rich, A.B. Hjelmeland, Glioma stem cell maintenance: The role of the microenvironment. Curr. Pharm. Des. 17, 2386–2401 (2011)
J. Kolenda, S.S. Jensen, C. Aaberg-Jessen, K. Christensen, C. Andersen, N. Brunner, B.W. Kristensen, Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J. Neuro-Oncol. 103, 43–58 (2011)
Z. Li, H. Wang, C.E. Eyler, A.B. Hjelmeland, J.N. Rich, Turning cancer stem cells inside out: An exploration of glioma stem cell signaling pathways. J. Biol. Chem. 284, 16705–16709 (2009)
R. Wang, K. Chadalavada, J. Wilshire, U. Kowalik, K.E. Hovinga, A. Geber, B. Fligelman, M. Leversha, C. Brennan, V. Tabar, Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468, 829–833 (2010)
M. Prinz, J. Priller, Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014)
M.B. Graeber, B.W. Scheithauer, G.W. Kreutzberg, Microglia in brain tumors. Glia 40, 252–259 (2002)
R.D. Leek, A.L. Harris, Tumor-associated macrophages in breast cancer. J. Mammary Gland Biol. Neoplasia 7, 177–189 (2002)
N.A. Charles, E.C. Holland, R. Gilbertson, R. Glass, H. Kettenmann, The brain tumor microenvironment. Glia 60, 502–514 (2012)
C.E. Green, T. Liu, V. Montel, G. Hsiao, R.D. Lester, S. Subramaniam, S.L. Gonias, R.L. Klemke, Chemoattractant signaling between tumor cells and macrophages regulates cancer cell migration, metastasis and neovascularization. PLoS One 4, e6713 (2009)
N.B. Hao, M.H. Lu, Y.H. Fan, Y.L. Cao, Z.R. Zhang, S.M. Yang, Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol. 2012, 948098 (2012)
E.A. Vasievich, L. Huang, The suppressive tumor microenvironment: A challenge in cancer immunotherapy. Mol. Pharm. 8, 635–641 (2011)
E.M. Dijkgraaf, M. Heusinkveld, B. Tummers, L.T. Vogelpoel, R. Goedemans, V. Jha, J.W. Nortier, M.J. Welters, J.R. Kroep, S.H. van der Burg, Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 73, 2480–2492 (2013)
K. Lolmede, L. Campana, M. Vezzoli, L. Bosurgi, R. Tonlorenzi, E. Clementi, M.E. Bianchi, G. Cossu, A.A. Manfredi, S. Brunelli, P. Rovere-Querini, Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J. Leukoc. Biol. 85, 779–787 (2009)
S. Bao, Q. Wu, S. Sathornsumetee, Y. Hao, Z. Li, A.B. Hjelmeland, Q. Shi, R.E. McLendon, D.D. Bigner, J.N. Rich, Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66, 7843–7848 (2006)
Y. Piao, J. Liang, L. Holmes, A.J. Zurita, V. Henry, J.V. Heymach, J.F. de Groot, Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neuro-Oncology 14, 1379–1392 (2012)
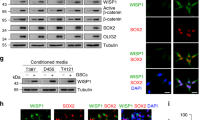
J. Yang, D. Liao, C. Chen, Y. Liu, T.H. Chuang, R. Xiang, D. Markowitz, R.A. Reisfeld, Y. Luo, Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells 31, 248–258 (2013)
J.J. Watters, J.M. Schartner, B. Badie, Microglia function in brain tumors. J. Neurosci. Res. 81, 447–455 (2005)
O.A. Guryanova, Q. Wu, L. Cheng, J.D. Lathia, Z. Huang, J. Yang, J. MacSwords, C.E. Eyler, R.E. McLendon, J.M. Heddleston, W. Shou, D. Hambardzumyan, J. Lee, A.B. Hjelmeland, A.E. Sloan, M. Bredel, G.R. Stark, J.N. Rich, S. Bao, Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell 19, 498–511 (2011)
C.L. Nilsson, R. Dillon, A. Devakumar, S.D. Shi, M. Greig, J.C. Rogers, B. Krastins, M. Rosenblatt, G. Kilmer, M. Major, B.J. Kaboord, D. Sarracino, T. Rezai, A. Prakash, M. Lopez, Y. Ji, W. Priebe, F.F. Lang, H. Colman, C.A. Conrad, Quantitative phosphoproteomic analysis of the STAT3/IL-6/HIF1alpha signaling network: An initial study in GSC11 glioblastoma stem cells. J. Proteome Res. 9, 430–443 (2010)
H. Wang, J.D. Lathia, Q. Wu, J. Wang, Z. Li, J.M. Heddleston, C.E. Eyler, J. Elderbroom, J. Gallagher, J. Schuschu, J. MacSwords, Y. Cao, R.E. McLendon, X.F. Wang, A.B. Hjelmeland, J.N. Rich, Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells 27, 2393–2404 (2009)
D.J. Brat, A.C. Bellail, E.G. Van Meir, The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-Oncology 7, 122–133 (2005)
A. Wu, J. Wei, L.Y. Kong, Y. Wang, W. Priebe, W. Qiao, R. Sawaya, A.B. Heimberger, Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncology 12, 1113–1125 (2010)
H. Zhang, W. Zhang, X. Sun, R. Dang, R. Zhou, H. Bai, J. Ben, X. Zhu, Y. Zhang, Q. Yang, Y. Xu, Q. Chen, Class A1 scavenger receptor modulates glioma progression by regulating M2-like tumor-associated macrophage polarization. Oncotarget 7, 50099–50116 (2016)
J.J. Letterio, A.B. Roberts, Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16, 137–161 (1998)
L. Gao, F. Li, B. Dong, J. Zhang, Y. Rao, Y. Cong, B. Mao, X. Chen, Inhibition of STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity, and induces mitochondria-dependent apoptosis in glioma cells. Int. J. Radiat. Oncol. Biol. Phys. 77, 1223–1231 (2010)
Y. Liu, C. Li, J. Lin, STAT3 as a therapeutic target for glioblastoma. Anti Cancer Agents Med. Chem. 10, 512–519 (2010)
D.S. Markovic, R. Glass, M. Synowitz, N. Rooijen, H. Kettenmann, Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J. Neuropathol. Exp. Neurol. 64, 754–762 (2005)
C. Gedye, L. Ailles, Isolation and characterization of cancer stem cells in vitro. Methods Mol. Biol. 946, 181–204 (2013)
S.C. Yu, Y.F. Ping, L. Yi, Z.H. Zhou, J.H. Chen, X.H. Yao, L. Gao, J.M. Wang, X.W. Bian, Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 265, 124–134 (2008)
D.R. Laks, M. Masterman-Smith, K. Visnyei, B. Angenieux, N.M. Orozco, I. Foran, W.H. Yong, H.V. Vinters, L.M. Liau, J.A. Lazareff, P.S. Mischel, T.F. Cloughesy, S. Horvath, H.I. Kornblum, Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells 27, 980–987 (2009)
K. Visnyei, H. Onodera, R. Damoiseaux, K. Saigusa, S. Petrosyan, D. De Vries, D. Ferrari, J. Saxe, E.H. Panosyan, M. Masterman-Smith, J. Mottahedeh, K.A. Bradley, J. Huang, C. Sabatti, I. Nakano, H.I. Kornblum, A molecular screening approach to identify and characterize inhibitors of glioblastoma stem cells. Mol. Cancer Ther. 10, 1818–1828 (2011)
A. Gray, T. Maguire, R. Schloss, M.L. Yarmush, Identification of IL-1beta and LPS as optimal activators of monolayer and alginate-encapsulated mesenchymal stromal cell immunomodulation using design of experiments and statistical methods. Biotechnol. Prog. 31, 1058–1070 (2015)
S.S. Hamed, C.M. Roth, Mathematical modeling to distinguish cell cycle arrest and cell killing in chemotherapeutic concentration response curves. J. Pharmacokinet. Pharmacodyn. 38, 385–403 (2011)
S. Bao, Q. Wu, R.E. McLendon, Y. Hao, Q. Shi, A.B. Hjelmeland, M.W. Dewhirst, D.D. Bigner, J.N. Rich, Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006)
J. Wei, J. Barr, L.Y. Kong, Y. Wang, A. Wu, A.K. Sharma, J. Gumin, V. Henry, H. Colman, R. Sawaya, F.F. Lang, A.B. Heimberger, Glioma-associated cancer-initiating cells induce immunosuppression. Clin. Cancer Res. 16, 461–473 (2010)
L. Benayoun and Y. Shaked, In vitro enrichment of tumor-initiating cells from human established cell lines. Curr. Protoc. Stem Cell Biol. Chapter 3, Unit 3.7 (2013)
E.H. Panosyan, D.R. Laks, M. Masterman-Smith, J. Mottahedeh, W.H. Yong, T.F. Cloughesy, J.A. Lazareff, P.S. Mischel, T.B. Moore, H.I. Kornblum, Clinical outcome in pediatric glial and embryonal brain tumors correlates with in vitro multi-passageable neurosphere formation. Pediatr. Blood Cancer 55, 644–651 (2010)
L. Yi, H. Xiao, M. Xu, X. Ye, J. Hu, F. Li, M. Li, C. Luo, S. Yu, X. Bian, H. Feng, Glioma-initiating cells: A predominant role in microglia/macrophages tropism to glioma. J. Neuroimmunol. 232, 75–82 (2011)
Y. Komohara, H. Horlad, K. Ohnishi, Y. Fujiwara, B. Bai, T. Nakagawa, S. Suzu, H. Nakamura, J. Kuratsu, M. Takeya, Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci. 103, 2165–2172 (2012)
T.O. Jensen, H. Schmidt, H.J. Moller, M. Hoyer, M.B. Maniecki, P. Sjoegren, I.J. Christensen, T. Steiniche, Macrophage markers in serum and tumor have prognostic impact in American joint committee on cancer stage I/II melanoma. J. Clin. Oncol. 27, 3330–3337 (2009)
H. Kurahara, H. Shinchi, Y. Mataki, K. Maemura, H. Noma, F. Kubo, M. Sakoda, S. Ueno, S. Natsugoe, S. Takao, Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J. Surg. Res. 167, e211–e219 (2011)
D. Niino, Y. Komohara, T. Murayama, R. Aoki, Y. Kimura, K. Hashikawa, J. Kiyasu, M. Takeuchi, N. Suefuji, Y. Sugita, M. Takeya, K. Ohshima, Ratio of M2 macrophage expression is closely associated with poor prognosis for Angioimmunoblastic T-cell lymphoma (AITL). Pathol. Int. 60, 278–283 (2010)
C. Steidl, T. Lee, S.P. Shah, P. Farinha, G. Han, T. Nayar, A. Delaney, S.J. Jones, J. Iqbal, D.D. Weisenburger, M.A. Bast, A. Rosenwald, H.K. Muller-Hermelink, L.M. Rimsza, E. Campo, J. Delabie, R.M. Braziel, J.R. Cook, R.R. Tubbs, E.S. Jaffe, G. Lenz, J.M. Connors, L.M. Staudt, W.C. Chan, R.D. Gascoyne, Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N. Engl. J. Med. 362, 875–885 (2010)
A. Sica, T. Schioppa, A. Mantovani, P. Allavena, Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 42, 717–727 (2006)
J. Lee, S. Kotliarova, Y. Kotliarov, A. Li, Q. Su, N.M. Donin, S. Pastorino, B.W. Purow, N. Christopher, W. Zhang, J.K. Park, H.A. Fine, Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9, 391–403 (2006)
X.Z. Ye, S.L. Xu, Y.H. Xin, S.C. Yu, Y.F. Ping, L. Chen, H.L. Xiao, B. Wang, L. Yi, Q.L. Wang, X.F. Jiang, L. Yang, P. Zhang, C. Qian, Y.H. Cui, X. Zhang, X.W. Bian, Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J. Immunol. 189, 444–453 (2012)
Z.G. Ouedraogo, J. Biau, J.L. Kemeny, L. Morel, P. Verrelle, E. Chautard, Role of STAT3 in genesis and progression of human malignant gliomas. Mol. Neurobiol. (2016). doi:10.1007/s12035-016-0103-0
K.M. Talasila, G.V. Rosland, H.R. Hagland, E. Eskilsson, I.H. Flones, S. Fritah, F. Azuaje, N. Atai, P.N. Harter, M. Mittelbronn, M. Andersen, J.V. Joseph, J.A. Hossain, L. Vallar, C.J. Noorden, S.P. Niclou, F. Thorsen, K.J. Tronstad, C. Tzoulis, R. Bjerkvig, H. Miletic, The angiogenic switch leads to a metabolic shift in human glioblastoma. Neuro. Oncol. 19, 383–393 (2016)
C. Villalva, S. Martin-Lanneree, U. Cortes, F. Dkhissi, M. Wager, A. Le Corf, J.M. Tourani, I. Dusanter-Fourt, A.G. Turhan, L. Karayan-Tapon, STAT3 is essential for the maintenance of neurosphere-initiating tumor cells in patients with glioblastomas: A potential for targeted therapy? Int. J. Cancer 128, 826–838 (2011)
M.W. Roomi, T. Kalinovsky, M. Rath, A. Niedzwiecki, Modulation of MMP-2 and MMP-9 secretion by cytokines, inducers and inhibitors in human glioblastoma T-98G cells. Oncol. Rep. 37, 1907–1913 (2017)
M.W. Roomi, T. Kalinovsky, J. Monterrey, M. Rath, A. Niedzwiecki, In vitro modulation of MMP-2 and MMP-9 in adult human sarcoma cell lines by cytokines, inducers and inhibitors. Int. J. Oncol. 43, 1787–1798 (2013)
E.S. Kim, Y.E. Choi, S.J. Hwang, Y.H. Han, M.J. Park, I.H. Bae, IL-4, a direct target of miR-340/429, is involved in radiation-induced aggressive tumor behavior in human carcinoma cells. Oncotarget 7, 86836–86856 (2016)
Acknowledgements
This work was supported by grants from the National Institutes of Health (2R01 EB008278-07) and the Rutgers Aresty Research Center. We thank Dr. Michael Masterman-Smith for the generous gift of patient-derived brain tumor stem cell lines, and we thank Jeffrey Barminko and Andrea Gray for gifts of PBMCs and guidance on their culture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 2574 kb)
Rights and permissions
About this article
Cite this article
Nusblat, L.M., Carroll, M.J. & Roth, C.M. Crosstalk between M2 macrophages and glioma stem cells. Cell Oncol. 40, 471–482 (2017). https://doi.org/10.1007/s13402-017-0337-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0337-5



