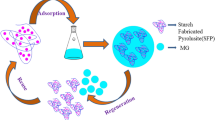
Abstract
Starch-g-poly(acrylic acid)/Pterocladia capillacea–derived activated carbon (St-g-P(AA)/P-AC) composites were prepared via aqueous solution graft copolymerization using starch, acrylic acid, and activated carbon of red alga Pterocladia capillacea (0–10%) with N,N′-methylenebisacrylamide crosslinker and ammonium persulfate (NH4)2S2O8 initiator. Fourier-transform infrared (FTIR) spectroscopy, Brunauer–Emmett–Teller (BET), X-ray diffraction (XRD), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and energy dispersive X-ray spectroscopy (EDX) were used to characterize St-g-P(AA)/P-AC composites. Additionally, St-g-P(AA)/P-AC composites were investigated for methylene blue (MB) dye removal from water. The impact of the beginning concentration of MB dye, temperature, pH, and adsorption time on MB dye removal was examined. The maximum adsorption capacity obtained at pH 8 was 496.29 mg/g at 0.02 mg/L composites dose and 100 mg/L MB dye. The properties of adsorption were studied by the adsorption isotherm, kinetic, and thermodynamic models. The pseudo-first-order and Freundlich isotherm models demonstrated the kinetics and equilibrium adsorptions data, respectively. The maximum monolayer capacity (qm) was 1428.57 mg/g from Langmuir isotherm. Thermodynamic parameters indicated that the MB dye adsorption is exothermic physisorption and spontaneous. The results show that St-g-P(AA)/P-AC composites were effective for MB dye adsorption from water solution and could be recycled.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The pollution produced by dyeing effluent has been judged to be a serious hazard to aquatic life since the textile effluents contain bright colour, high chemical oxygen demand (COD), low biochemical oxygen demand (BOD), acidic pH, and other harmful pollutants [1,2,3,4,5,6,7]. Dye substances are hazardous to human health because they have the potential to produce mutagenesis and carcinogenesis [8, 9]. Dye substances also interfere with the natural biological processes in water bodies. There is substantial evidence that dyes harm the liver and the central nervous system [9,10,11].
Methylene blue (MB), a basic cationic dye, officially named 3,7-bis(dimethylamino)-phenothiazin-5-ium chloride, has been extensively used in several applications such as dyeing cotton, paper, wool, silk, jute, and leather due to its low price and wide availability [12,13,14]. The MB dye structure’s unique blue and red color in aqueous solution and toluene, respectively, due to electrons delocalization from the conjugated π-bonds (chromophore group) are present [12, 15]. To date, there are various methods have been investigated for removing MB dye from water resources, such as coagulation and flocculation [16, 17], electrochemical [18], photocatalysis [19, 20], membrane filtration [21, 22], anaerobic or aerobic biodegradation treatment [23, 24], and adsorption [25,26,27,28]. The adsorption process is the most popular technique among these approaches. It has attracted a lot of interest because of its efficient procedures, high efficiency, lack of requirement for a vast application area, low costs, and absence of toxicity and bad consequences [14, 29,30,31]. Two crucial characteristics of an adsorption process are high adsorption and separation performance [26, 32]. As a result, creating a novel adsorbent with a high adsorption capacity is an exciting endeavor.
Recently, various developments in polymeric adsorbents have focused on using natural polysaccharides as treatment agents to eliminate water contamination [33, 34]. Due to their non-toxic nature and biodegradability, starch [35], chitosan [36], and gum [37] receive special consideration for usage as adsorbents in wastewater treatment. Starch is an abundant biopolymer derived from biodegradable agriculture, a low-cost material used especially to prepare green composites [38]. Starch can be chemically modified through oxidation, hydrolysis, esterification, etherification, and grafting, which can significantly improve the characteristics of the starch and allow it to be used for water treatment [9, 39]. The chemical grafting of vinyl monomer poly (acrylic acid) PAA onto starch (St) has increased much attention. Starch-grafted-poly(acrylic acid) (St-g-P(AA)) are becoming more significant due to their potential applications in the environment, agriculture, and biomedical [40]. It has been used as flocculants, ion exchangers, hydrogels, and super-absorbents, among other applications [41].
The development of St-g-P(AA) superabsorbent composites has been recognized. The addition of some additives or fillers into the St-g-P(AA) such as mica [42], attapulgite [43], kaolinite [44], Zeolite [45, 46], metals nanoparticles, or cellulose nanowhiskers [12], for instance, have been utilized in polymers preparation to achieve lower costs of production and higher properties. The study of the literature reveals that no comprehensive studies have been conducted on the application of the P-AC on St-g-P(AA) in the removal of MB dye from aqueous solution and the recovery qualities.
This study reports the synthesis of composites as a super-adsorbent through the graft copolymerization reaction of starch and acrylic acid in the presence of Pterocladia capillacea–driven AC. The cross-linker used in this process was N,N-methylenebisacrylamide (NMBA), and the initiator was ammonium persulfate (APS). FT-IR, scanning electron microscopy (SEM), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX), surface area analysis, and thermal gravimetric analysis (TGA) were all used to describe the superabsorbent composites that were created. On the St-g-P(AA)/P-AC composite, the adsorption equilibrium of a cationic dye called methylene blue (MB) dye from artificial wastewater was examined in detail. To study adsorption isotherms, kinetics, and thermodynamics, the specific impacts that various parameters have on adsorption, such as pH, beginning dye concentration, contact time, adsorbent dose, and temperature, were investigated. Experiments involving adsorption and desorption were also carried out to determine whether the prepared composite could be reused.
2 Experimental
2.1 Materials
Acrylic acid (AA, Assy = 99%) was obtained from Merck Schuchardt OHG (Germany). Ammonium persulfate ((NH4)2S2O8, Assy = 98%) was obtained from Oxford Lab Chem (Navghar Road, INDIA). N,N′-methylenebisacrylamide (MBA, Assy = 99%) were obtained from SIGMA (USA). Starch (Assy = 99.97%) was obtained from Qualikems Fine Chem Pvt. Ltd. (Nandesari, Vadodara, India), and Methylene blue (MB, Assy = 99%) from Honeywell Riedel-de Haën AG (SEELZE-HANNOVER, Germany) (Fig. 1). Hydrochloric acid (HCl, Assy = 30–34%) was obtained from SD Fine-Chem Limited (Mumbai, India). Sodium hydroxide (NaOH, Assy = 96%) was purchased from El Nasr Pharmaceutical Chemical Company (ADWIC, Egypt). Pterocladia capillacea was collected from the Mediterranean coast in Alexandria, Egypt.
2.1.1 Synthesis of activated carbon from red algae Pterocladia capillacea
Red algae Pterocladia capillacea was cleaned using distilled water, dried in an oven, milled, crushed, and stored until used. Activated carbon from Pterocladia capillacea (P-AC) was synthesized by the stated activated method [47]. Briefly, the dried Pterocladia capillacea 10 g was soaked with a solution of 5 g ZnCl2 for 24 h. Afterwards, the combinations were heated to 110 °C for 24 h to remove excess water. Then, they were carbonized in a quartz tube with a holding time of 30 min at a temperature of 600 °C. After the activated carbon that had been obtained had been cooled to room temperature, washed with boiling water, and oven dried at 70 °C, it was subjected to a 2-h reflux in a soxhelt containing one million parts hydrochloric acid, after which it was filtered and washed with distilled water until the pH was neutral. The final result of P-AC was dried at 70 °C, then crushed and sieved to a particle size of less than 60 μm, and then it was stored in a dark glass bottle until it was needed.
2.2 Synthesis of St-g-P(AA)/P-AC composites
At 85 °C, starch (1 g) was distributed in distilled water (50 mL) and agitated for 30 min under an N2 environment before cooling to 50 °C. To create free radicals, 1.0 g ammonium persulfate was added to the reaction mixture and agitated for 15 min [12]. Then, to complete the grafting reaction, precise amounts of (acrylic acid) AAc (7 g), MBA (1.0 g), and P-AC (0–10 wt % in relation to the sum weight of starch and AAc) were added to the reaction mixture and heated to 70 °C with stirring for 3 h. The St-g-P(AA)/PC-AC (0–10%) composites were cooled to room temperature, filtered, and then extensively washed with distilled water to eliminate unreacted compounds. Totally, composites were crushed, sieved after being oven-dried to a consistent weight, and sieved to smaller than 100 µm.
2.3 Characterization
FTIR analysis was made using a VERTEX70 Spectrometer connected to platinum ATR unit model V-100, Germany. FTIR analysis was employed at 400 and 4000 cm–1 wavenumber range to study the formed chemical bonds and functional groups. The Brunner Emmett Teller (BET) surface areas were determined by N2 adsorption–desorption isotherm using BELSORP-Mini II BEL equipment, Japan. Thermal analyzes were carried out using the SDT650-Simultaneous Thermal Analyzer device, USA, at a temperature range of 50 to 1000 °C, at a temperature increase rate of 5 °C/min. X-ray diffraction (XRD) patterns were obtained on a Bruker’s X-ray diffractometer, Germany, (second generation Bruker 2D Phaser, X-ray diffractograms (XRD) run at 30 kV, in the range 2θ: 5–80°, with Cu Kα radiation (λ = 1.540598 Å). The morphological structure was performed using Scan Electron Microscope and Energy Dispersive X-ray Spectroscopy using JEOL-JSM-5300 LV, Tokyo, Japan. UV–visible spectra of MB dye before and after adsorption were recorded on PG spectrophotometer instrument model T80, UK. The point of zero charge (pHpzc) was determined as described by Shoaib et al. (2020). In brief, in nine flasks, 50 mg of St-g-P(AA)/P-AC adsorbent was added to a 50 mL 0.1 M NaNO3 solution. The pH (pHi) of the starting solution was changed from 2 to 10 by adding 0.1 M HCl or NaOH. The final pH (pHf) of the supernatant liquid was measured, and the difference between the beginning and final pHs (Δ pH = pHi – pHf) was plotted against the initial pH (pHi) [48]. The point where the curve intersected the x-axis is the composite’s pHZPC value [49] Bench-top pH/mV/temperature meter model JENCO-6173 was used for pH value adjustment. Bench-top laboratory incubator shaker JSSI-100 T JS Research Inc., Korea, was used for temperature effect studies.
2.4 MB dye adsorption and desorption tests
The adsorption study evaluated the MB dye removal capacity of the St-g-P(AA)/P-AC composites. Briefly, in a run of 250 mL conical flask, 20 mg of adsorbent was shaken in 100 mL of MB dye solution. The experimental design was done under shaking (ca. 200 rpm) at a controlled temperature (25 ± 2 °C). At chosen time intervals, a supernatant aliquot was collected and determined at λmax 665 nm. Using a plot of the calibration curve have linear regression equation (R2 > 0.996) the MB dye solution concentrations were measured before and after adsorption. The capacity of adsorption of the St-g-P(AA)/P-AC samples for MB dye was obtained from the following Eq. (1):
where qe is the MB dye adsorption capacity loaded adsorbent (mg/g), C0 and Ce represent the MB dye beginning and equilibrium concentrations (mg/L), m (g) is the amount of St-g-P(AA)/P-AC and V (L) is the MB dye solution volume.
Similar procedures were used to assess how specific parameters might affect the adsorption capacity: contact time (0 to120 min), pH values (3 to 10), dose (5 to 25 mg for 100 mL dye solution), MB dye solution beginning concentration (60 to 140 mg/L), and temperature range (25 to 40 °C).
Desorption experiments: the samples consumed in the adsorption experiments with a MB dye solution beginning concentration (100 mg/L) and 15 mg St-g-P(AA)/P-AC dose were collected and then dried at 70 °C. The MB dye loaded on the adsorbent samples was desorbed using 0.1 M HCl as an eluent. 15 mg of MB dye-loaded composites was obscured in elution media (100 mL) under shaking at 25 ± 1 °C for 2 h. The eluted MB dye per gram of adsorbent (qdes) from the concentration of MB desorbed (Cdes) in the solution was calculated by using the following Eq. (2):
where V is the volume of the eluent used in Liters and m is the weight of the adsorbent in grams. Desorption % was calculated by comparing the MB dye desorbed (qdes) to the MB adsorbed (q) using this Eq. (3):
For regeneration and desorption experiments, samples were shaken in 0.1 M NaOH solution for 30 min and cleaned several times with distilled water. It was necessary to carry out this process to deprotonate the functional groups that had been formed due to the desorption step. To determine whether or not the composites have a chance of functioning effectively as practical MB dye adsorbents, samples were subjected to three successive adsorption–desorption cycles.
Error analysis functions coefficient of determination (R2), and average percentage errors (APE%) function were applied to investigate the power of the isotherm models fitted by the nonlinear regression method [50]. The Average percentage errors (APE%) were determined using Eq. (4):
3 Results and discussion
3.1 Characterization
3.1.1 FTIR analysis
FTIR spectra were displayed for P-AC, St-g-P(AA)/P-AC0%, and St-g-P(AA)/P-AC7.5%, respectively (Fig. 2). For P-AC, the bands at 3795 and 3195 cm–1 represent bonding and non-bonding OH groups on their surface [51]. Figure 2a shows these band locations of aldehydes, ketones, quinones, and carboxylated groups which can all be identified by the peak of C = O that is located at 1590 cm–1 [52, 53]. In addition, there were peaks at 1190 cm–1 that represented C–O stretching vibrations [54], and bands at 886–756 cm–1 indicated H2PO4–, PO42–, disulfide, or aromatic structures. In the meantime, the band at a frequency of 467 cm–1 may indicate metal oxide or aromatic structures.
Starch spectra Fig. 2b showed O–H stretching peak at 3290, C–H symmetric stretching peak at 2926, and C = O stretching peak at 1641 cm–1. At 1148 and 997 cm–1, the C–O–C triplet band stretching absorption was also visible. New peaks developed in the spectrum after the grafting of acrylic acid [St-g-P(AA)/P-AC0%] (Fig. 2c). These peaks included an acrylic C = O stretching band at 1709 cm–1, acrylic acid symmetric and asymmetric COO stretching at 1541 and 1444 cm–1 and a weak peak at 3187 cm–1 owing to water molecules. The peak at 2935 cm–1 is due to C–H symmetric stretching. The grafting of acrylic acid onto starch was validated by this knowledge. This confirmation is backed up by previous reports of starch graft copolymerization with acrylic acid [44, 55]. FTIR spectra for St-g-P(AA)/P-AC7.5% (Fig. 2d) and St-g-P(AA)/P-AC0% (Fig. 2c) were similar. FTIR peaks intensities are very small when compared to intensities of the St-g-P(AA), which explains the similarity of the figures between St-g-P(AA)/P-AC7.5% and St-g-P(AA)/P-AC0% (Figs. 2c and 2d). The present at 1700, 1540, and 1443 cm–1 were assigned to C = O– and COO–, which corroborated the composite’s formation.
3.1.2 Pore structure analysis
Figure 3a displays the adsorption–desorption isotherms of nitrogen at 77 K on St, St-g-P(AA)/P-AC0%, St-g-P(AA)/P-AC5%, St-g-P(AA)/P-AC7.5%, and St-g-P(AA)/P-AC10%. According to the IUPAC categorization [56], a type III isotherm with notable H3 hysteresis loops suggests that the pores are of low energy, homogeneous solid surface that possesses mesoporosity. As long as the interactions between the adsorbent and the adsorbate are modest, it can be found in mesoporous or microporous adsorbents. In addition, the BJH desorption pore size distributions are displayed in Fig. 3b. These distributions include St, St-g-P(AA)/P-AC0 percent, St-g-P(AA)/P-AC5%, St-g-P(AA)/P-AC7.5%, and St-g-P(AA)/P-AC10%. It can be noted that the pores between 2 and 30 nm were dominant for all; that is, both mesopores and macropores are present [57].
The porous structure parameters of St, St-g-P(AA)/P-AC0%, St-g-P(AA)/P-AC5%, St-g-P(AA)/P-AC7.5%, and St-g-P(AA)/P-AC10% are summarized in Table 1.
3.1.3 Thermal analysis
Thermal properties of the St-g-P(AA)/P-AC0%, and St-g-P(AA)/P-AC7.5% were studied, and their corresponding thermograms are exemplified in, Fig. 4(a, b) and Table 2. Both St-g-P(AA)/P-AC0%, and St-g-P(AA)/P-AC7.5% composite showed three stages of decomposition, and the total weight loss was 78.92 and 77.12%, respectively, which indicated that the presence of P-AC in the network of the composite led to an increase in thermal stability. The initial weight loss occurs due to the hydrophilic polymer’s water loss [58]. The second and third weight loss is due to the degradation of starch in graft copolymer [59] and polymer chain and matrix [31], respectively.
3.1.4 XRD analysis
The XRD results of P-AC, St, St-g-PAA/P-AC0%, and St-g-PAA/P-AC7.5% were presented in Fig. 5. There were sharp peaks in XRD patterns of pure starch (St) and P-AC. Afterwards, graft copolymerization St-g-PAA, the sample displayed a broad peak without characteristics peaks, which indicated that the crystal structure of the starch was damaged [55] and confirmed its amorphous nature [26]. Similar results were also reported by Saberi et al. (2019) and Shi et al. (2012) [26, 55]. The peak intensity of the original properties of P-AC was diminished as a result of its incorporation into the polymerization process, which caused damage to the peaks’ original forms.
3.1.5 SEM and EDX analyses
The surface morphological features of St-g-P(AA)/P-AC0%, St-g-P(AA)/P-AC7.5%, and MB- St-g-P(AA)/P-AC7.5% were examined by SEM study. A very rough surface and appearance of voids were cleared for St-g-P(AA)/P-AC0% composite (Fig. 6a). When P-AC was incorporated (Fig. 6b), St-g-P(AA)/P-AC7.5% the surface roughness was expressively decreased due to the partial fill-up of the voids by P-AC a smooth surface was appeared compared to untreated composite (Fig. 6a). This morphological analysis revealed that P-AC was successfully incorporated into the polymeric matrix. After the MB dye adsorption on St-g-P(AA)/P-AC7.5% (Fig. 6c), the morphology of the composite has significantly changed. It should be observed that after MB adsorption, the composite became rougher.
Figure 7 shows the elemental composition of St-g-P(AA)/P-AC0%, and St-g-P(AA)/P-AC7.5% adsorbents, it was calculated that St-g-P(AA)/P-AC0, and St-g-P(AA)/P-AC7.5 contained about 40.95 and 42.24 wt % of carbon, 52.19 and 50.69 wt % of oxygen, 4.44 and 3.91 wt % of nitrogen, and 2.41 and 3.15 wt % of sulfur, respectively. Sulfur and Nitrogen are predicted in the EDX spectra of St-g-P(AA)/P-AC0% comes from APS, and MBA, which were the free radical initiator and cross-linked in the polymerization reaction, respectively. The wt % of sulfur increased due to the presence of sulfur in the P-AC as described in Shoaib et al. (2020) [47].
3.1.6 Points of zero charge (pHPZC)
The electrical charge nature in the adsorbent and adsorbed particles directly influences the physical adsorption process reported for St-g-P(AA)/P-AC7.5% composites. Hence, pHPZC gives useful information on adsorption of St-g-P(AA)/P-AC7.5% at specific conditions of pH [12]. The pHPZC is the pH required to affect a net zero charge on a solid surface if there isn’t any particular sorption. The pHPZC of adsorbent St-g-P(AA)/P-AC7.5 surface was found to be about 2.3 (Fig. 8). So, it can be assumed that when pH > 2.3 the surface will be charged negative, and so favor cationic MB dye specimens adsorption [60].
3.2 Sorption study
Utilizing a variety of thermodynamic and kinetic analyses, such as the impact of pH, composite mass, beginning MB dye concentrations, the effect of temperature and thermodynamics, and other significant factors, an evaluation of the rate and adsorption capacity of MB dye from aqueous solution is carried out. This evaluation determines how well the MB dye can remove itself from the solution.
3.2.1 Impact of P-AC addition to St-g-P(AA) on MB dye adsorption at different pH
The impact of the addition P-AC to St-g-P(AA) 0–10% on removal of MB dye was tested at [MB] = 100 mg/L, pH = 8, dose = 20 mg, contact time = 120 min, and shacking speed = 200 rpm. Figure 9 shows that P-AC has some effect on MB dye adsorption capacity. The maximum MB adsorption capacity increased with an increase in %P-AC to 496.29 mg/g obtained by St-g-P(AA)/P-AC7.5%, which increased by 30 mg/g after adding 7.5% of P-AC then slightly decreased after addition 10% of P-AC. This can be explained by the matrix’s greater porosity as a result of the presence of P-AC, facilitating interaction between both polymeric chains and MB molecules. The enhancement of crosslinking spots within the matrix is responsible for the small decrease in MB adsorption property [61, 62]. The hydroxyl groups of P-AC engage strongly with the carboxylic groups of PAAc, as previously observed [55, 56], reducing the number of carboxylate groups accessible to interact with MB dye molecule due to hydrogen bonds [62,63,64]. Overall, these answers indicate that all of the composites have excellent MB dye removal properties, which is critical for treating wastewater contaminated with dyes. The composite which has the maximum amount of MB dye adsorption was used to perform the batch experiment.
3.2.2 Influence of pH
As presented in Fig. 10, the MB dye adsorption on St-g-P(AA)/P-AC7.5% as a function of pH at [[MB] = 100 mg/L, dose = 20 mg, temperature = 25 ± 2 °C, shacking = 200 rpm, and contact time = 120 min] increases with the increasing of the pH where, the maximum and minimum capacity of adsorption reaches 353.81 and 496.29 mg/g at pH 3 and 8, respectively. The weakened MB dye adsorption removal % reaches around 70% at pH 3 and pH 4 due to the H+ ions contend with the cationic MB dye molecules for sites of adsorption, and the absence of electrostatic attraction due to most portions of composite carboxylic groups is in ‒COOH protonated form, also, reduction anionic sites on the adsorbent surface. Otherwise, when a solution pH of ≥ 5, the MB adsorption removal % reaches 90–99% at pH 8 where H+ions and MB molecules had no competition for the carboxylated groups of PAAc, favouring MB adsorption. In addition, the excessive number of ‒COO− ions contained by the composite creates repulsive forces, which cause the polymeric matrixes to expand. Overall, matrix expansion increases MB adsorption and causes liquid uptake [12]. Such results suggest strongly that composite St-g-P(AA)/P-AC7.5% as proficient MB adsorbents in a pH wide range (pH 5–9). Therefore, pH 8 was used as the preferred pH for all the experiments’ work due to achieving maximum adsorption capacity.
3.2.3 Influence of initial MB dye concentration
Adsorption studies were operated at beginning concentrations of MB dye ranging from 60 to 140 mg/L with St-g-P(AA)/P-AC7.5% doses (0.005, 0.01, 0.015, 0.02 and 0.025 g/L) (Fig. 11). The data referred that the increased MB dye adsorption capacity of St-g-P(AA)/P-AC7.5% increases the beginning concentration of MB dye. The adsorption capacity of St-g-P(AA)/P-AC7.5% at dose 0.005 g/L-0.025 g/L increased from 1114.59 to 1482.00 mg/g and 238.55 to 511.91 mg/g, respectively, when the MB dye primary concentration increases from 60 to 140 mg/L. On the other hand, the MB dye removal percent was inversely proportional with MB dye concentrations, which clearly suggested that MB dye adsorption from its aqueous solution was dependent on its primary concentration in addition to a reduction of active surface size with an increase in MB dye concentration [53].
3.2.4 Impact of contact time
MB dye removal by St-g-P(AA)/P-AC7.5% at different shaking intervals was estimated by consuming MB dye with concentrations of 60, 80, 100, 120, and 140 mg/L. Figure 12 shows the concentration variations of MB dye in external solution in reference to the primary concentration vs time. The concentration of MB dye in the external solution decreases until it reaches equilibrium. As a result of being adsorbed in the composite, the concentration of MB dye in the solution dropped. After achieving the equilibrium (50 min for concentration 60, 80,100 mg/L), 60 min for concentration 120, 140 mg/L)), the concentration of MB dye in the solution is stable and the St-g-P(AA)/P-AC7.5% gets saturated (Ct/C0 = 0.02). Desorption may occur with the passage of time, implying that the composite may progressively desorb a limited number of adsorbed MB dye back to the solution [40].
3.2.5 Effect of St-g-P(AA)/P-AC7.5% dose
The effect of the adsorbent dose of St-g-P(AA)/P-AC7.5% on MB dye adsorption was performed by changing the adsorbent dose (0.005 to 0.025 g/L) and five different initial MB dye concentrations from 60 to 140 mg/ L at pH 8 (Fig. 13). It is observed that the increase in the amount of the hydrogel St-g-P(AA)/P-AC7.5% resulted in a rise in MB removal with a maximum of 99.40%. So, there is a decrease in the equilibrium adsorption capacity (qe) of MB dye. Raising the sorbent dosage increased the amount of sorbent functional groups, surface area, and pore volume available for MB dye adsorption on the St-g-P(AA)/P-AC7.5% surface may be able to explain this outcome. This finding is in line with previous studies [65, 66].
3.2.6 Adsorption kinetics
The kinetic adsorption data of MB dye onto St-g-P(AA)/P-AC7.5% were processed to understand and predict the nature of the process of adsorption. Table 3 summarized the three linear equations of kinetic models that had been employed to understand the reaction mechanism [67,68,69], i.e., pseudo-first-order (PFO) Eq. (5), pseudo-second-order (PSO) Eq. (6), and intraparticle diffusion (IPD) models Eq. (7). Kinetics parameters, coefficients of determination R2, and the statistic average percentage errors APE%, were summarized in Tables 4 and 5 for different initial MB dye and St-g-P(AA)/P-AC7.5% concentrations. Figure 14a, b displayed the PFO and PSO kinetics models of MB dye adsorption onto St-g-P(AA)/P-AC7.5%. Table 4 shows that (A) for the PFO kinetic model, the qe(exp) values are in agreement with the predicted qe(th) values more than the PSO. (B) The rate constant of PFO adsorption increases with increased MB dye concentration and St-g-P(AA)/P-AC7.5% doses. (C) R2 values are greater than 0.990 for PFO kinetics model. So, the PFO kinetic model is more adequate to describe the adsorption process of MB dye onto St-g-P(AA)/P-AC7.5%. Douven et al. (2015) and Tan and Hameed (2017) [70, 71] defined the range of PFO models validity only under either of these two sets of conditions: (i) control of the reaction and Henry regime adsorption or (ii) control of the reaction and high dose of adsorbent.
Intraparticle diffusion is the process of components transfer from the solution bulk to the solid phase. Figure 15c reveals that the IPD adsorption plots are not linear throughout the entire time range selected and could be divided into three linear regions. The three regions were completed within 20, 60, and 120 min. According to the research that has been conducted, the first region would be characterized by the external surface adsorption, in which the MB dye molecules diffuse through the bulk solution to the external surface of the adsorbents, or the boundary layer diffusion of MB dye molecules [72], with the second region being dominated by the IPD. The decrease in the concentration of MB dye in the aqueous phase, as well as the reduction in the number of active sites that are available for adsorption, causes the IPD to begin to slow down in the final region [73]. This is due to the fact that the last region also contains fewer active sites. There was not a single plot of IPD that crossed the origin, which indicates that intraparticle diffusion was not only a rate-controlling step but also participated in the process C1,2,3,0 (Fig. 14c). Table 5 displays the values of kdif and C, as well as the correlation coefficients, for each of the three locations in which the linear fitting of the kinetic data was performed. Where for the second region, the values of kdif are directly proportional to MB concentration and inversely proportional with St-g-P(AA)/P-AC7.5% doses. Also, the values of C are considered directly proportional with St-g-P(AA)/P-AC7.5% doses and inversely proportional with MB concentration. A negative C value could be clarified by the dual impacts of both film diffusion and surface reaction control [71, 74]. By comparing the statistic average percentage errors APE% values for the data presented in Tables 4 and 5, the PFO order was lower than PSO and IPD model, thereby indicating that PFD kinetic model verdicts the adsorption rate of MB dye onto St-g-P(AA)/P-AC7.5% under the studied conditions.
3.2.7 Adsorption isotherm, distribution coefficient, and average percentage errors analysis (APE %)
Adsorption isotherms were applied to explain the adsorption result by Langmuir [75], Freundlich [76], Temkin [77], and Dubinin–Radushkevich (D–R) isotherms [78,79,80] for MB dye concentration of 60–140 mg/L onto adsorbed St-g-P(AA)/P-AC7.5% (0.005 – 0.025 g/L) (Fig. 15). Table 6 illustrates the details of each linearized isotherm models and the associated parameters.
Based on the correlation coefficient (R2) values and the low error value (APE%), it can be assumed that the best isotherms model fitness arrangement for adsorptive removal of MB dye by St-g-P(AA)/P-AC7.5% as Freundlich isotherm < Dubinin-Radushkevich > Langmuir < Temkin. According to the Langmuir model, the adsorption of MB dye takes place on a homogenous surface by monolayer adsorption, and no interaction takes place between the different adsorbed species [81]. The maximum monolayer capacity (qm) was 1428.57 mg/g at a dose of 0.005 g/L. On calculating the separation factor, RL using followed equation [RL = 1/(1 + KaCo)]. Commonly, the value of RL > 1 adsorption is unfavorable, RL = 1 adsorption is linear, 0 < RL < 1 adsorption is favorable, and RL = 0 adsorption is irreversible [14]. It was further confirmed that the isotherm is favorable, as RL value equals 0.0367.
Meanwhile, Freundlich isotherm [76] suggested the adsorption process of MB dye based on a sorption heterogeneous surface. The value of n provides an indication of the degree to which there is a non-linear relationship between the concentration of the solution and adsorption as follows: If the value of n is equal to one, then the adsorption process is linear; if n is less than one, then the adsorption process is chemical; and if n is greater than one, then the adsorption process is a beneficial physical process [50]. The n value was greater than unity, signifying that the adsorption process is favorable with higher values of n indicating the adsorption process is physical.
The Temkin isotherm model [77] makes the assumption that the heat of adsorption of all the molecules in the layer decreases linearly with coverage due to adsorbent–adsorbate interactions and that the adsorption is characterized by a uniform distribution of binding energies, up to some maximum binding energy. This model also assumes that the heat of adsorption of all the molecules in the layer decreases linearly with coverage due to adsorbent–adsorb. This model also operates under the premise that the adsorption process is typified by a distribution of binding energies that is consistent throughout. The Temkin sorption potentials, denoted by letter A, are 898.88 L/mg, while the heat of adsorption, denoted by letter b, is 18.44 J. The low b values that were reported in this experiment imply that the sorbent and the sorbate do not interact strongly with one another.
On the other hand, the D–R isotherm was also utilized to evaluate the porosity of apparent free energy and the adsorption characteristics [78,79,80]. It is possible to apply it to the explanation of adsorption on surfaces that are either homogenous or heterogenous [82]. The maximum capacity qm obtained for the adsorption of MB dye is 1724.17 mg/g which is higher than the qm obtained from the Langmuir isotherm model. The values of E calculated are 0.0694–2.6726 kJ/ mol. Ion-exchange processes tend to have bonding energies that fall within the range of 8–16 kJ/mol on average, denoting physical processes play a significant role in the adsorption process of MB dye by the St-g-P(AA)/P-AC7.5% composite. Freundlich, Temkin, and D-R isotherm models basically stand true for the adsorption phenomenon observed for St-g-P(AA)/P-AC7.5%.
3.2.8 Thermodynamic study
MB dye removal % onto St-g-P(AA)/P-AC7.5% as a reaction temperature function was valued in the temperature ranges 25–40 °C to determine the thermodynamic parameters such as Gibbs free energy (ΔG, J/mol), enthalpy change (ΔH, J/mol) and entropy change (ΔS, J/K mol) using Van’t Hoff equations (Eqs. (12, 13, 14)) [83]:
where Kd is the distribution coefficient (Kd = qe/Ce, L/g), and T (K) and R (8.314 J/mol K) are the absolute temperature and the universal gas constant, respectively. The values of ΔH and ΔS were determined based on the plot of ln Kd vs 1/T, and a summary of their findings can be found in Table 7. According to Table 7, all of the ∆G° values were negative, and their absolute values decreased as the temperature increased; as a result, the MB dye adsorption process on St-g-P(AA)/P-AC7.5% was an unintentional one. The presence of an exothermic process was substantiated by the fact that the MB dye adsorption on St-g-P(AA)/P-AC7.5% had a negative 19.6909 kJ/mol value for the ΔH° parameter. The value of the change in enthalpy, denoted by ΔH°, was less than 84 kJ/mol, which indicates that physisorption was the process that took place [84, 85]. During the process of adsorption, the value of entropy, denoted by the symbol ∆S°, was found to be negative (–12.1401 J/K.mol), which reflects a decrease in the amount of randomness at the interface between the solid and the solution.
3.2.9 Mechanism of adsorption.
Figure 16 displays the FTIR spectra of the adsorbents, St-g-P(AA)/P-AC7.5%, which were acquired before and after the adsorption trials. This method is appropriate for gaining an understanding of the mechanism underlying MB adsorption [12]. The FTIR spectra of MB-St-g-P(AA)/P-AC7.5% (Fig. 16) showed that the bands of St-g-P(AA)/P-AC7.5% at 3187, 1700, 1540, 1163, 1041, and 510 cm–1 are blue and moved into 3228, 1707, 1543, 1167, 1045, and 522 cm–1, respectively, accompanied by intensity decreasing; as a result of MB dye adsorption, new peaks at 2109, 1989, 1593, 1489, 1388, 1327, 1238, 1135, and 441 cm–1 appeared. Such behavior originated due to the formation of (1) bonds between the sulfur atoms in MB dye heterocycles and the OH groups found in the St-g-P(AA)/P-AC7.5%; (2) hydrogen bonds between the negatively charged groups in the St-g-P(AA)/P-AC7.5% and unsaturated MB dye dimethylamino groups. To finish, the band allocated to the vibration modes attributed to methylene groups (at 2934 cm–1) moved to 2930 cm−1, indicating that hydrophobic contacts were engaged in addition to electrostatic interactions between MB and St-g-P(AA)/P-AC7.5%. As a consequence, the phenomenon known as MB adsorption in the St-g-P(AA)/P-AC7.5% is assumed to be caused by a combination of electrostatic interactions, hydrogen bonds, and hydrophobic interactions.
3.2.10 Regeneration studies
To discover the economic feasibility and reusability of St-g-P(AA)/P-AC7.5% adsorbents, desorption studies of MB from loaded St-g-P(AA)/P-AC7.5% adsorbents were performed using 0.1 N HCl as an eluted medium. So, ~ 60% of the MB previously adsorbed onto was desorbed after three cycles. As shown in Fig. 17a, the maximum desorption (ca. 67.23%) was verified for the first use of the adsorbents.
Three consecutive adsorption/desorption cycles with the regenerated St-g-P(AA)/P-AC7.5% composite were performed. The amount of adsorption presented remained steady throughout the cycles, with only a minor drop (1%) after three cycles. (Fig. 17b), indicating that it might be utilized as a long-term MB removal method. This finding is in line with previous studies [62] where it experienced only a slight drop after regeneration cycles.
3.2.11 Analysis of the outcomes of the current investigation in comparison to those of earlier studies
To justify the viability of the prepared St-g-P(AA)/P-AC7.5% as effective adsorbents for MB dye removal at different conditions, the adsorption capacity was compared with different adsorbents (Table 8). The prepared composite showed highly adsorption capacity equal to 1482 mg/g than other adsorbents, followed by Freundlich isotherm, pseudo-first-order kinetic model, and adsorption was exothermic.
4 Conclusion
Starch-g-poly(acrylic acid)/Pterocladia capillacea–activated carbon (St-g-PAA/P-AC) composites were prepared and characterized by FTIR, BET, XRD, TGA, EDX, and SEM. In addition, we describe the removal of MB dye from artificial wastewater using St-g-PAA/P-AC composites. The maximum MB dye adsorption capacity achieved at composites dose 0.02 mg/L, MB dye (100 mg/L), pH 8, 120 min obtained with the composite at 7.5 wt.% of P-AC, 496.29 mg/g. Batch experimental for MB dye adsorption operated at MB dye beginning concentration (60–140 mg/L), temperature (25–40 °C), pH (3–10) and contact time (0–120 min). The adsorption isotherms data were arranged for MB dye adsorptive removal by St-g-P(AA)/P-AC7.5% as Freundlich isotherm < Dubinin-Radushkevich > Langmuir > Temkin based on the coefficient of determination values (R2) and the low value of APE%. The maximum monolayer capacity (qm) was 1428.57 mg/g at a dose of 0.005 g/L from Langmuir isotherm. The adsorption kinetics data were correlated with pseudo-first-order with R2 values greater than 0.990 model where qe(exp) values are in agreement with the predicted qe(th) values more than the PSO. Thermodynamic parameters (ΔG°, ΔH°, and ΔS°) showed that the MB dye adsorption occurred physisorption, exothermic, and spontaneously in nature. Based on these results, the prepared St-g-PAA/P-AC composites can effectively be considered potential adsorbents to MB dye removal from industrial effluent and have good recycling capability.
Data availability
The datasets used in this investigation are accessible for review upon request from the corresponding author of the paper.
References
Salem DMS, Khaled A, El Nemr A (2013) Assessment of pesticides and polychlorinated biphenyls (PCBs) in sediments of the Egyptian Mediterranean Coast. Egypt J Aquat Res 39(2):141–152. https://doi.org/10.1016/j.ejar.2013.11.001
Salem DMSA, El Sikaily A (2014) El Nemr A (2014) Organocholorines and their risk in marine shellfish collected from the Mediterranean coast. Egypt Egypt J Aquat Res 40:93–101. https://doi.org/10.1016/j.ejar.2014.03.004
Ragab S, El Sikaily A, El Nemr A (2016) Concentrations and Sources of Pesticides and PCBs in Surficial Sediments of the Red Sea Coast. Egypt Egypt J Aquat Res 42:365–374
Hassaan MA, El Nemr A (2020) Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt J Aquat Res 46(3):207–220. https://doi.org/10.1016/j.ejar.2020.08.007
Salem DMSA, Morsy FA-EM, El Nemr A, El-Sikaily A, Khaled A (2014) The monitoring and risk assessment of aliphatic and aromatic hydrocarbons (PAHs) in sediments of the Red Sea. Egypt Egyptn J Aquat Res 40:333–348. https://doi.org/10.1016/j.ejar.2014.11.003
El Nemr A, Moneer AA, Ragab S, El Sikaily A (2016) Distribution and sources of n-alkanes and polycyclic aromatic hydrocarbons in Shellfish of the Egyptian Red Sea coast. Egypt J Aquat Res 42:121–131. https://doi.org/10.1016/j.ejar.2016.05.003
El Nemr A, Shoaib AG, El Sikaily A, Ragab S, Mohamed AEDA, Hassan AF (2021) Utilization of green alga Ulva lactuca for sustainable production of meso-micro porous nano activated carbon for adsorption of Direct Red 23 dye from aquatic environment. Carbon Letters, 1–16.
Sivaraj R, Namasivayam C, Kadirvelu K (2001) Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions. Waste Manage 21(1):105–110
Al E, Güçlü G, İyim TB, Emik S, Özgümüş S (2008) Synthesis and properties of starch-graft-acrylic acid/Na-montmorillonite superabsorbent nanocomposite hydrogels. J Appl Polym Sci 109(1):16–22
Kadirvelu K, Palanival M, Kalpana R, Rajeswari SJBT (2000) Activated carbon from an agricultural by-product, for the treatment of dyeing industry wastewater. Bioresour Tech 74(3):263–265
Nasr MF, Abo El-Ola SM, Ramadan A, Hashem A (2006) A comparative study between the adsorption behavior of activated carbon fiber and modified alginate I. Basic dyes adsorption Polym-Plast Tech Eng 45(3):335–340
Gomes RF, de Azevedo ACN, Pereira AG, Muniz EC, Fajardo AR, Rodrigues FH (2015) Fast dye removal from water by starch-based nanocomposites. J Coll Interf Sci 454:200–209
Rawat AP, Kumar V, Singh DP (2019) A combined effect of adsorption and reduction potential of biochar derived from mentha plant waste on removal of methylene blue dye from aqueous solution. Sep Sci Technol 55:907–921. https://doi.org/10.1080/01496395.2019.1580732
El Nemr A, Shoaib AG, El Sikaily A, Mohamed AEDA, Hassan AF (2021) Evaluation of cationic Methylene Blue dye removal by high surface area mesoporous activated carbon derived from Ulva lactuca. Environ Proces 8(1):311–332
Mills A, Hazafy D, Parkinson J, Tuttle T, Hutchings MG (2011) Effect of alkali on methylene blue (CI Basic Blue 9) and other thiazine dyes. Dyes Pigm 88(2):149–155
Mahecha FC, Parra DC, Sandoval JA, Mayorga MA, Ortiz SA (2021) Removal of cationic dyes utilizing natural coffee pulp coagulant. Chem Eng Trans 87:487–492
Mahmoud MS, Farah JY, Farrag TE (2013) Enhanced removal of methylene blue by electrocoagulation using iron electrodes. Egypt J Pet 22:211–216
Yu Y (2021) Ag-doped ZnO nanorods synthesized on porous Si electrode using a facile electrochemical approach for removal of Methylene Blue from wastewater. Inter J Electrochem Sci 16(2).
Balasurya S, Das A, Alyousef AA, Alqasim A, Almutairi N, Khan SS (2021) Facile synthesis of Bi2MoO6-Ag2MoO4 nanocomposite for the enhanced visible light photocatalytic removal of methylene blue and its antimicrobial application. J Mol Liqu 337:116350
Kamaraj M, Srinivasan NR, Assefa G, Adugna AT, Kebede M (2020) Facile development of sunlit ZnO nanoparticles-activated carbon hybrid from pernicious weed as an operative nano-adsorbent for removal of methylene blue and chromium from aqueous solution: extended application in tannery industrial wastewater. Environ Technol Innovat 17:100540
Daud NA, Shamsuddin MR, Pradanawati SA (2021) Synthesis and characterization of fly ash-based geopolymer membrane for Methylene Blue dye removal. In IOP Conference Series: Earth and Environ Sci (Vol. 765, No. 1, p. 012081). IOP Publishing.
Asman S, Yusof NA, Abdullah AH, Haron MJ (2012) Synthesis and characterization of hybrid molecularly imprinted polymer (MIP) membranes for removal of methylene blue (MB). Molecules 17:1916–1928. https://doi.org/10.3390/molecules17021916
Zamel D, Khan AU (2021) Bacterial immobilization on cellulose acetate based nanofibers for methylene blue removal from wastewater: Mini-review. Inorganic Chem Commun 108766.
Gaur N, Narasimhulu K, PydiSetty Y (2018) Recent advances in the bio-remediation of persistent organic pollutants and its effect on environment. J Clean Prod 198:1602–1631
El Nemr A, Shoaib AG, El Sikaily A, Mohamed AEDA, Hassan AF (2021) Evaluation of cationic Methylene Blue dye removal by high surface area mesoporous activated carbon derived from Ulva lactuca. Environ Process 8(1):311–332
Saberi A, Alipour E, Sadeghi M (2019) Superabsorbent magnetic Fe3O4-based starch-poly (acrylic acid) nanocomposite hydrogel for efficient removal of dyes and heavy metal ions from water. J Polym Res 26(12):1–14
Durrani WZ, Nasrullah A, Khan AS, Fagieh TM, Bakhsh EM, Akhtar K, Khan SB, Din IU, Khan MA, Bokhari A (2022) Adsorption efficiency of date palm based activated carbon-alginate membrane for methylene blue. Chemosphere 302:134793. https://doi.org/10.1016/j.chemosphere.2022.134793
Xue H, Wang X, Xu Q, Dhaouadi F, Sellaoui L, Seliem MK, Lamine AB, Belmabrouk H, Bajahzar A, Bonilla-Petriciolet A, Li Z, Li Q (2022) Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: a comparative study by experimental and advanced modeling analysis. Chem Eng J 430:132801
Mauter MS, Zucker I, Perreault F, Werber JR, Kim JH, Elimelech M (2018) The role of nanotechnology in tackling global water challenges. Nature Sustainability 1(4):166–175
Pang X, Sellaoui L, Franco D, Dotto GL, Georgin J, Bajahzar A, Belmabrouk H, Lamine AB, Bonilla-Petriciolet A, Li Z (2019) Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem Eng J 378:122101
Li Z, Sellaoui L, Dotto GL, Lamine AB, Bonilla-Petriciolet A, Hanafy H, Belmabrouk H, Netto MS, Erto A (2019) Interpretation of the adsorption mechanism of Reactive Black 5 and Ponceau 4R dyes on chitosan/polyamide nanofibers via advanced statistical physics model. J Mol Liq 285:165–170
Li JJ, Wang CC, Fu HF, Cui JR, Xu P, Guo J, Li JR (2017) High-performance adsorption and separation of anionic dyes in water using a chemically stable graphene-like metal–organic framework. Dalton Trans 46(31):10197–10201
Khan AS, Ibrahim TH, Khamis MI, Nancarrow P, Iqbal J, AlNashef I, Jabbar NA, Hassan MF, Mjalli FS (2021) Preparation of sustainable activated carbon-alginate beads impregnated with ionic liquid for phenol decontamination. J Clean Prod 321:128899. https://doi.org/10.1016/j.jclepro.2021.128899
Jamshaid A, Iqbal J, Hamid A, Ghauri M, Muhammad N, Nasrullah A, Rafiq S, Shah NS (2019) Fabrication and evaluation of cellulose-alginate-hydroxyapatite beads for the removal of heavy metal ions from aqueous solutions. Z Phys Chem 233(9):1351–1375. https://doi.org/10.1515/zpch-2018-1287
Mittal H, Alhassan SM, Ray SS (2018) Efficient organic dye removal from wastewater by magnetic carbonaceous adsorbent prepared from corn starch. J Environ Chem Eng 6(6):7119–7131
Yan H, Yang H, Li A, Cheng R (2016) pH-tunable surface charge of chitosan/graphene oxide composite adsorbent for efficient removal of multiple pollutants from water. Chem Eng J 284:1397–1405
Sharma G, Kumar A, Devi K, Sharma S, Naushad M, Ghfar AA, Stadler FJ (2018) Guar gum-crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Inter J Biol Macromol 114:295–305
Ruiz-Hitzky E, Darder M, Aranda P, Ariga K (2010) Advances in biomimetic and nanostructured biohybrid materials. Adv Mater 22(3):323–336
Mostafa KM (1997) Synthesis of poly (acrylamide)-starch and hydrolyzed starch graft copolymers as a size base material for cotton textiles. Polym Degrad Stability 55(2):125–130
Baghbadorani NB, Behzad T, Etesami N, Heidarian P (2019) Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly (acrylic acid) superadsorbent hydrogels. Composites Part B: Eng 176:107084
Celik M, Sacak M (2002) Synthesis and characterization of starch-poly (methyl methacrylate) graft copolymers. J Appl Polym Sci 86(1):53–57
Lee WF, Chen YC (2005) Effect of intercalated reactive mica on water absorbency for poly (sodium acrylate) composite superabsorbents. European Polym J 41(7):1605–1612
Li A, Zhang J, Wang A (2007) Utilization of starch and clay for the preparation of superabsorbent composite. Bioresour Tech 98(2):327–332
Kabiri K, Zohuriaan-Mehr MJ (2003) Superabsorbent hydrogel composites Polym Adv Tech 14(6):438–444
Zhang Y, Zhao L, Chen Y (2017) Synthesis and characterization of starch-g-Poly (acrylic acid)/Organo-Zeolite 4 A superabsorbent composites with respect to their water-holding capacities and nutrient-release behavior. Polym Compos 38(9):1838–1848
Zhang Y, Zhao L, Chen Y (2016) Swelling properties and environmental responsiveness of superabsorbent composite based on starch-G-poly acrylic acid/organo-zeolite. J Macromol Sci, Part B 55(7):662–679
Shoaib AGM, El-Sikaily A, El Nemr A, Mohamed AEDA, Hassan AF (2020) Preparation and characterization of highly surface area activated carbons followed type IV from marine red alga (Pterocladia capillacea) by zinc chloride activation. Biomass Conver Bioref. https://doi.org/10.1007/s13399-020-00760-8
Shoaib AG, El-Sikaily A, El Nemr A, Mohamed AEDA, Hassan AA (2020). Testing the carbonization condition for high surface area preparation of activated carbon following type IV green alga Ulva lactuca. Biomass Conversion and Biorefinery, pp.1–16.
Alamin NU, Khan AS, Nasrullah A, Iqbal J, Ullah Z, Din IU, Muhammad N, Khan SZ (2021) Activated carbon-alginate beads impregnated with surfactant as sustainable adsorbent for efficient removal of methylene blue. Int J Biol Macromol 176:233–243. https://doi.org/10.1016/j.ijbiomac.2021.02.017
El Nemr A, El-Sikaily A, Khaled A (2010) Modeling of adsorption isotherms of Methylene Blue onto rice husk activated carbon. Egypt J Aquat Res 36(3):403–425
El-Sikaily A, El Nemr A, Khaled A (2011) Copper sorption onto dried red alga Pterocladia capillacea and its activated carbon. Chem Eng J 168:707–714
Oliveira LCA, Pereira E, Guimaraes IR, Vallone A, Pereirac M, Mesquita JP, Sapag K (2009) Preparation of activated carbons from coffee husks utilizing FeCl3and ZnCl2 as activating agents. J Hazard Mater 165:87–94
El Nemr A, El-Sikaily A, Khaled A, Abdelwahab O (2015) Removal of toxic chromium from aqueous solution, wastewater and saline water by marine red alga Pterocladia capillacea and its activated carbon. Arab J Chem 8(1):105–117
Kılıç M, Apaydın-Varol E, Pütün AE (2012) Preparation and surface characterization of activated carbons from Euphorbia rigida by chemical activation with ZnCl2, K2CO3, NaOH and H3PO4. Appl Surf Sci 261:247–254
Zhang Y, Gao P, Zhao L, Chen Y (2016) Preparation and swelling properties of a starch-g-poly (acrylic acid)/organo-mordenite hydrogel composite. Front Chem Sci Eng 10(1):147–161
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting Physisorption data for gas/solid interface with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Barrett E P, Joyner L G, Halenda P P, Amer J (1951) The determination of pore volume and area distributions in porous substances. I. Computations from Nitrogen Isotherms, Chem. Soc., 73, 373.
Pathania D, Sharma R, Kalia S (2012) Graft copolymerization of acrylic acid onto gelatinized patato starch for removal of metal ions and organic dyes from aqueous system. Adv Mater Lett 3:259–264
Czarnecka E, Nowaczyk J (2020) Semi-natural superabsorbents based on starch-g-poly (acrylic acid): modification, synthesis and application. Polym 12(8):1794
Vieira MGA, de Almeida Neto AF, Carlos da Silva MG, Nobrega CC, Melo Filho AA (2012) Characterization and use of in natura and calcined rice husks for biosorption of heavy metals ions from aqueous effluents. Braz J Chem Eng 29(3):619–633
Wang L, Zhang JP, Wang AQ (2008) Removal of methylene blue from aqueous solution using chitosan-g-poly (acrylic acid)/montmorillonite super adsorbent nanocomposite. Coll Surf A 322(1–3):47–53
de Azevedo AC, Vaz MG, Gomes RF, Pereira AG, Fajardo AR, Rodrigues FH (2017) Starch/rice husk ash based superabsorbent composite: high methylene blue removal efficiency. Iran Polym J 26(2):93–105
Spagnol C, Rodrigues FHA, Neto A, Pereira AGB, Fajardo AR, Radovanovic E, Rubira AF, Muniz EC (2012) Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur Polym J 48(3):454–463
Eldeeb TM, El Nemr A, Khedr MH, El-Dek SI (2021) Novel bio-nanocomposite for efficient copper removal. Egypt J Aquat Res 47(3):261–267. https://doi.org/10.1016/j.ejar.2021.07.002
Nasrullah A, Bhat AH, Naeem A, Isa MH, Danish M (2018) High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int J Biological Macromolecules 107:1792–1799. https://doi.org/10.1016/j.ijbiomac.2017.10.045
Shoaib AG, El Nemr A, El Sikaily A, Masoud MS, Ramadan MS (2022) Amidoxime modification of polyacrylonitrile/Pterocladia capillacea-derived activated carbon composite for adsorption of toxic chromium from aquatic environment. Carbon Letters 32(2):513–535
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens, Handlingar 24:1–39
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanity Eng Div Am Soc Civil Eng 89:31–59
Srinivasan K, Balasubramanian N, Ramakrishan TV (1988) Studies on chromium removal by rice husk carbon. Ind J Environ Health 30:376–387
Douven S, Paez CA, Gommes CJ (2015) The range of validity of sorption kinetic models. J Coll Interf Sci 448:437–450
Tan KL, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48
Mohammed N, Grishkewich N, Berry R, Tam K (2015) Cellulose nanocrystal-alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 22(6):3725–3738
Dragan ES, Loghin DFA, Cocarta AI (2014) Efficient sorption of Cu2+ by composite chelating sorbents based on potato starch-graft polyamidoxime embedded in chitosan beads. ACS Appl Mater Interf 6:16577–16592. https://doi.org/10.1021/am504480q|
Rudzinski W, Plazinski W (2008) Kinetics of solute adsorption at solid/solution interfaces: on the special features of the initial adsorption kinetics. Langmuir 24(13):6738–6744
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Freundlich H (1906) Über die adsorption in Lösungen. Z Phys Chem 57:385–470
Tempkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:217–222
Radushkevich LV (1949) Potential theory of sorption and structure of carbons. Zh Fiz Khim 23:1410–1420
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem Rev 60:235–266
Dubinin MM (1965) Modern state of the theory of volume filling of micropore adsorbents during adsorption of gases and steams on carbon adsorbents. Zh Fiz Khim 39:1305–1317
Kumarathilaka P, Jayaweera V, Wijesekara H, Kottegoda IRM, Rosa SRD, Vithanage M (2016) Insights into starch coated nanozero valent iron-graphene composite for Cr (VI) removal from aqueous medium. J Nanomater.
Shahwan T, Erten HN (2004) Temperature effects on barium sorption on natural kalinite and chlorite-illite clays. J Radioanal Nucl Chem 260(1):43–48
Krug R, Hunter W, Grieger R (1976) Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of van’t Hoff and Arrhenius data. J Phys Chem 80:2335–2341
Faust SD, Aly OM (1987) Adsorption processes for water treatment, Butterworth London.
Benhouria A, Islam MA, Zaghouane-Boudiaf H, Boutahala M, Hameed BH (2015) Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chem Eng J. https://doi.org/10.1016/j.cej.2015.02.030
Liu Y, Zheng Y, Wang A (2010) Enhanced adsorption of Methylene Blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J Environ Sci 22(4):486–493
Atta A, Akl MA, Youssef AM, Ibraheim MA (2013) Superparamagnetic core-shell polymeric nanocomposites for efficient removal of methylene blue from aqueous solutions. Adsor Sci Tech 31(5):397–419
Wang Y, Wang W, Wang A (2013) Efficient adsorption of methylene blue on an alginate-based nanocomposite hydrogel enhanced by organo-illite/smectite clay. Chem Eng J 228:132–139
Chen RP, Zhang YL, Shen LF, Wang XY, Chen JQ, Ma AJ, Jiang WM (2015) Lead(II) and methylene blue removal using a fully biodegradable hydrogel based on starch immobilized humic acid. Chem Eng J 268:348–355
Wang F, Chang PR, Zheng PW, Ma XF (2015) Monolithic porous rectorite/starch composites: fabrication, modification and adsorption. Appl Surface Sci 349:251–258
Acknowledgements
The Egyptian Science and Technological Development Fund (STDF) provided partial funding for this study, which the corresponding author is grateful for (Project No. CB-4874 and CB-22816).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Thank you to the Egyptian Science and Technology Development Fund (STDF) for providing partial financial support for this project (Project No. CB-4874 and CB-22816).
Author information
Authors and Affiliations
Contributions
The practical portion was led by Dr. Amany G. M. Shoaib, who also wrote the first draft. Professor Dr. Ahmed El Nemr oversaw the actual work, edited the article, and then submitted it. The work was overseen by Professor Mamdouh S. Masoud, Professor Mohamed S. Ramadan, Amany El Sikaily, and Dr. Safaa Ragab.
Corresponding author
Ethics declarations
Consent to participate.
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shoaib, A.G.M., Sikaily, A.E., Ragab, S. et al. Starch-grafted-poly(acrylic acid)/Pterocladia capillacea–derived activated carbon composite for removal of methylene blue dye from water. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03382-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03382-4