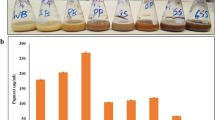
Abstract
In this study, organic acids present in dark fermentative cheese whey effluent (DFCWE) were utilized to produce biological hydrogen via photo fermentation by R. sphaeroides O.U.001 cells in 2-L double-walled cylindrical PBR with a working volume of 1.5 L. Plackett-Burman design-based analysis revealed organic acid concentration (OA), temperature, and light intensity as the most significant variables. Experiments were performed at different conditions of (OA, 8–16 g L−1), temperature (25–37 °C), and light intensity (8–12 klx). Optimum values were obtained by Box-Behnken design matrix (BBD) based on the impact on hydrogen production rate (HPR) and under optimum values (OA concentration, 12 g L−1; temperature, 31 °C; and light intensity, 10 klx); HPR of 41.94 mL L−1 h−1 was obtained, which lies in close proximity with the predicted production rate of 41.65 mL L−1 h−1 with the correlation coefficient (R2) and coefficient of variance as 0.9801 and 0.0521, respectively. PBR performance for treating DFCWE was checked by performing mathematical modelling using four models. Kinetic study of DFCWE consumption and growth profile of the bacterial cell were investigated by fitting experimental values into Monod and logistic equations, respectively. Parameters of the modified Gompertz equation and Luedeking-Piret models gave proper simulated fitting with experimental H2 production obtained under optimized bioprocess variables. Metabolite analysis revealed that acetic and lactic acids were utilized to produce biohydrogen under uncontrolled pH. Findings of the current investigation could be a promising strategy for obtaining better hydrogen productivity in photo fermentation.








Similar content being viewed by others
References
Arregi A, Amutio M, Lopez G, Bilbao J, Olazar M (2018) Evaluation of thermochemical routes for hydrogen production from biomass: a review. Energy Convers Manag 165:696–719. https://doi.org/10.1016/j.enconman.2018.03.089
Li Y, Zhang Z, Zhang Q, Tahir N, Jing Y, Xia C, Zhu S, Zhang X (2020) Enhancement of bio-hydrogen yield and pH stability in photo fermentation process using dark fermentation effluent as succedaneum. Bioresour Technol 297:122–504. https://doi.org/10.1016/j.biortech.2019.122504
Basak N, Jana AK, Das D, Saikia D (2014) Photofermentative molecular biohydrogen production by purple-non-sulfur (PNS) bacteria in various modes: the present progress and future perspective. Int J Hydrog Energy 39:6853–6871. https://doi.org/10.1016/j.ijhydene.2014.02.093
Zhang T, Jiang D, Zhang H, Jing Y, Tahir N, Zhang Y, Zhang Q (2020) Comparative study on bio-hydrogen production from corn stover: photo-fermentation, dark-fermentation and dark-photo co-fermentation. Int J Hydrog Energy 45:3807–3814. https://doi.org/10.1016/j.ijhydene.2019.04.170
Phan PT, Nguyen B-S, Nguyen T-A, Kumar A, Nguyen V-H (2020) Lignocellulose-derived monosugars: a review of biomass pre-treating techniques and post-methods to produce sustainable biohydrogen. Biomass Convers Biorefin:1–15. https://doi.org/10.1007/s13399-020-01161-7
Dębowski M, Korzeniewska E, Filipkowska Z, Zieliński M, Kwiatkowski R (2014) Possibility of hydrogen production during cheese whey fermentation process by different strains of psychrophilic bacteria. Int J Hydrog Energy 39:1972–1978. https://doi.org/10.1016/j.ijhydene.2013.11.082
Ghimire A, Luongo V, Frunzo L, Pirozzi F, Lens PN, Esposito G (2017) Continuous biohydrogen production by thermophilic dark fermentation of cheese whey: use of buffalo manure as buffering agent. Int J Hydrog Energy 42:4861–4869. https://doi.org/10.1016/j.ijhydene.2016.11.185
M’Arimi M, Kiprop A, Ramkat R, Kiriamiti H (2020) Progress in applications of advanced oxidation processes for promotion of biohydrogen production by fermentation processes. Biomass Convers Biorefin:1–25. https://doi.org/10.1007/s13399-020-01019-y
Rao R, Basak N (2020) Optimization and modelling of dark fermentative hydrogen production from cheese whey by Enterobacter aerogenes 2822. Int J Hydrog Energy 46:1777–1800. https://doi.org/10.1016/j.ijhydene.2020.10.142
Baeyens J, Zhang H, Nie J, Appels L, Dewil R, Ansart R, Deng Y (2020) Reviewing the potential of bio-hydrogen production by fermentation. Renew Sust Energ Rev 131:110023. https://doi.org/10.1016/j.rser.2020.110023
Bundhoo ZM (2019) Potential of bio-hydrogen production from dark fermentation of crop residues: a review. Int J Hydrog Energy 44:17346–17362. https://doi.org/10.1016/j.ijhydene.2018.11.098
Bao M, Su H, Tan T (2013) Dark fermentative bio-hydrogen production: effects of substrate pre-treatment and addition of metal ions or L-cysteine. Fuel 112:38–44. https://doi.org/10.1016/j.fuel.2013.04.063
Rao R, Basak N (2020) Development of novel strategies for higher fermentative biohydrogen recovery along with novel metabolites from organic wastes: the present state of the art. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1964
Osman AI, Deka TJ, Baruah DC, Rooney DW (2020) Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers Biorefin:1–19. https://doi.org/10.1007/s13399-020-00965-x
Argun H, Kargi F (2011) Bio-hydrogen production by different operational modes of dark and photo-fermentation: an overview. Int J Hydrog Energy 36:7443–7459. https://doi.org/10.1016/j.ijhydene.2011.03.116
Uyar B, Eroglu I, Yücel M, Gündüz U (2009) Photofermentative hydrogen production from volatile fatty acids present in dark fermentation effluents. Int J Hydrog Energy 34:4517–4523. https://doi.org/10.1016/j.ijhydene.2008.07.057
Koku H, Eroğlu İ, Gündüz U, Yücel M, Türker L (2002) Aspects of the metabolism of hydrogen production by Rhodobacter sphaeroides. Int J Hydrog Energy 27:1315–1329. https://doi.org/10.1016/S0360-3199(02)00127-1
Argun H, Kargi F (2010) Photo-fermentative hydrogen gas production from dark fermentation effluent of ground wheat solution: effects of light source and light intensity. Int J Hydrog Energy 35:1595–1603. https://doi.org/10.1016/j.ijhydene.2009.12.040
Das SR, Basak N (2020) Molecular biohydrogen production by dark and photo fermentation from wastes containing starch: recent advancement and future perspective. Bioprocess Biosyst Eng 44:1–25. https://doi.org/10.1007/s00449-020-02422-5
Basak N, Das D (2007) The prospect of purple non-sulfur (PNS) photosynthetic bacteria for hydrogen production: the present state of the art. World J Microbiol Biotechnol 23:31–42. https://doi.org/10.1007/s11274-006-9190-9
Hitam C, Jalil A (2020) A review on biohydrogen production through photo-fermentation of lignocellulosic biomass. Biomass Convers Biorefin:1–19. https://doi.org/10.1007/s13399-020-01140-y
Sybounya S, Nitisoravut R (2020) Hybrid composite of modified commercial activated carbon and Zn-Ni hydrotalcite for fermentative hydrogen production. J Environ Chem Eng:104801. https://doi.org/10.1016/j.jece.2020.104801
Castillo-Moreno P, Serrato JC, Willison JC, Magnin J-P (2018) Photohydrogen production from lactose and lactate by recombinant strains of Rhodobacter capsulatus: modeling and optimization. Int J Hydrog Energy 43:21231–21245. https://doi.org/10.1016/j.ijhydene.2018.09.038
Dolly S, Pandey A, Pandey BK, Gopal R (2015) Process parameter optimization and enhancement of photo-biohydrogen production by mixed culture of Rhodobacter sphaeroides NMBL-02 and Escherichia coli NMBL-04 using Fe-nanoparticle. Int J Hydrog Energy 40:16010–16020. https://doi.org/10.1016/j.ijhydene.2015.09.089
Laocharoen S, Reungsang A (2014) Isolation, characterization and optimization of photo-hydrogen production conditions by newly isolated Rhodobacter sphaeroides KKU-PS5. Int J Hydrog Energy 39:10870–10882. https://doi.org/10.1016/j.ijhydene.2014.05.055
Li Y, Zhang Z, Jing Y, Ge X, Wang Y, Lu C, Zhou X, Zhang Q (2017) Statistical optimization of simultaneous saccharification fermentative hydrogen production from Platanus orientalis leaves by photosynthetic bacteria HAU-M1. Int J Hydrog Energy 42:5804–5811. https://doi.org/10.1016/j.ijhydene.2016.11.182
Liu H, Zhang Z, Zhang Q, Tahir N, Jing Y, Li Y, Lu C (2019) Optimization of photo fermentation in corn stalk through phosphate additive. Bioresour Technol Rep 7:100278. https://doi.org/10.1016/j.biteb.2019.100278
Valentin JD, Qin X-H, Fessele C, Straub H, van der Mei HC, Buhmann MT, Maniura-Weber K, Ren Q (2019) Substrate viscosity plays an important role in bacterial adhesion under fluid flow. J Colloid Interface Sci 552:247–257. https://doi.org/10.1016/j.jcis.2019.05.043
Asunis F, De Gioannis G, Isipato M, Muntoni A, Polettini A, Pomi R, Rossi A, Spiga D (2019) Control of fermentation duration and pH to orient biochemicals and biofuels production from cheese whey. Bioresour Technol 289:121722. https://doi.org/10.1016/j.biortech.2019.121722
Chandrasekhar K, Lee Y-J, Lee D-W (2015) Biohydrogen production: strategies to improve process efficiency through microbial routes. Int J Mol Sci 16:8266–8293. https://doi.org/10.3390/ijms16048266
Özgür E, Uyar B, Öztürk Y, Yücel M, Gündüz U, Eroğlu I (2010) Biohydrogen production by Rhodobacter capsulatus on acetate at fluctuating temperatures. Resour Conserv Recycl 54:310–314. https://doi.org/10.1016/j.resconrec.2009.06.002
Lazaro CZ, Varesche MBA, Silva EL (2015) Effect of inoculum concentration, pH, light intensity and lighting regime on hydrogen production by phototrophic microbial consortium. Renew Energy 75:1–7. https://doi.org/10.1016/j.renene.2014.09.034
Basak N, Jana AK, Das D (2014) Optimization of molecular hydrogen production by Rhodobacter sphaeroides OU 001 in the annular photobioreactor using response surface methodology. Int J Hydrog Energy 39:11889–11901. https://doi.org/10.1016/j.ijhydene.2014.05.108
Androga DD, Sevinç P, Koku H, Yücel M, Gündüz U, Eroglu I (2014) Optimization of temperature and light intensity for improved photofermentative hydrogen production using Rhodobacter capsulatus DSM 1710. Int J Hydrog Energy 39:2472–2480. https://doi.org/10.1016/j.ijhydene.2013.11.114
Al-Mohammedawi HH, Znad H, Eroglu E (2018) Synergistic effects and optimization of photo-fermentative hydrogen production of Rhodobacter sphaeroides DSM 158. Int J Hydrog Energy 43:15823–15834. https://doi.org/10.1016/j.ijhydene.2018.06.140
Shi X-Y, Li W-W, Yu H-Q (2014) Optimization of H2 photo-fermentation from benzoate by Rhodopseudomonas palustris using a desirability function approach. Int J Hydrog Energy 39:4244–4251. https://doi.org/10.1016/j.ijhydene.2014.01.016
Mishra P, Singh L, Ab Wahid Z, Krishnan S, Rana S, Islam MA, Sakinah M, Ameen F, Syed A (2018) Photohydrogen production from dark-fermented palm oil mill effluent (DPOME) and statistical optimization: renewable substrate for hydrogen. J Clean Prod 199:11–17. https://doi.org/10.1016/j.jclepro.2018.07.028
Wang R, Wen H, Cui C (2019) Bio-hydrogen production by a new isolated strain Rhodopseudomonas sp. WR-17 using main metabolites of three typical dark fermentation type. Int J Hydrog Energy 44:25145–25150. https://doi.org/10.1016/j.ijhydene.2019.04.143
Lin R, Cheng J, Yang Z, Ding L, Zhang J, Zhou J, Cen K (2016) Enhanced energy recovery from cassava ethanol wastewater through sequential dark hydrogen, photo hydrogen and methane fermentation combined with ammonium removal. Bioresour Technol 214:686–691. https://doi.org/10.1016/j.biortech.2016.05.037
Nasr M, Tawfik A, Ookawara S, Suzuki M, Kumari S, Bux F (2015) Continuous biohydrogen production from starch wastewater via sequential dark-photo fermentation with emphasize on maghemite nanoparticles. J Ind Eng Chem 21:500–506. https://doi.org/10.1016/j.jiec.2014.03.011
Moreno R, Escapa A, Cara J, Carracedo B, Gómez X (2015) A two-stage process for hydrogen production from cheese whey: integration of dark fermentation and biocatalyzed electrolysis. Int J Hydrog Energy 40:168–175. https://doi.org/10.1016/j.ijhydene.2014.10.120
Rai PK, Singh S, Asthana R (2012) Biohydrogen production from cheese whey wastewater in a two-step anaerobic process. Appl Biochem Biotechnol 167:1540–1549. https://doi.org/10.1007/s12010-011-9488-4
Alvarez-Guzmán CL, Cisneros-de la Cueva S, Balderas-Hernández VE, Smoliński A, De León-Rodríguez A (2020) Biohydrogen production from cheese whey powder by Enterobacter asburiae: effect of operating conditions on hydrogen yield and chemometric study of the fermentative metabolites. Energy Rep 6:1170–1180. https://doi.org/10.1016/j.egyr.2020.04.038
Seifert K, Waligorska M, Laniecki M (2010) Hydrogen generation in photobiological process from dairy wastewater. Int J Hydrog Energy 35:9624–9629
Rai PK, Asthana R, Singh S (2014) Optimization of photo-hydrogen production based on cheese whey spent medium. Int J Hydrog Energy 39:7597–7603. https://doi.org/10.1016/j.ijhydene.2013.09.011
Fang F, Mu Y, Sheng G-P, Yu H-Q, Li Y-Y, Kubota K, Harada H (2013) Kinetic analysis on gaseous and aqueous product formation by mixed anaerobic hydrogen-producing cultures. Int J Hydrog Energy 38:15590–15597. https://doi.org/10.1016/j.ijhydene.2013.03.157
Nath K, Das D (2011) Modeling and optimization of fermentative hydrogen production. Bioresour Technol 102:8569–8581. https://doi.org/10.1016/j.biortech.2011.03.108
Rasdi Z, Mumtaz T, Hassan MA (2012) Kinetic analysis of biohydrogen production from anaerobically treated POME in bioreactor under optimized condition. Int J Hydrog Energy 37:17724–17730. https://doi.org/10.1016/j.ijhydene.2012.08.095
Blanco V, Oliveira G, Zaiat M (2019) Dark fermentative biohydrogen production from synthetic cheese whey in an anaerobic structured-bed reactor: performance evaluation and kinetic modeling. Renew Energy 139:1310–1319. https://doi.org/10.1016/j.renene.2019.03.029
Sharma Y, Li B (2009) Optimizing hydrogen production from organic wastewater treatment in batch reactors through experimental and kinetic analysis. Int J Hydrog Energy 34:6171–6180. https://doi.org/10.1016/j.ijhydene.2009.06.031
Koku H, Eroǧlu İ, Gündüz U, Yücel M, Türker L (2003) Kinetics of biological hydrogen production by the photosynthetic bacterium Rhodobacter sphaeroides OU 001. Int J Hydrog Energy 28:381–388. https://doi.org/10.1016/S0360-3199(02)00080-0
Biebl H, Pfennig N (1981) Isolation of members of the family Rhodospirillaceae. The prokaryotes Springer, Isolation of Members of the Family Rhodospirillaceae
Basak N, Jana AK, Das D (2016) CFD modeling of hydrodynamics and optimization of photofermentative hydrogen production by Rhodopseudomonas palustris DSM 123 in annular photobioreactor. Int J Hydrog Energy 41:7301–7317. https://doi.org/10.1016/j.ijhydene.2016.02.126
Nath K, Muthukumar M, Kumar A, Das D (2008) Kinetics of two-stage fermentation process for the production of hydrogen. Int J Hydrog Energy 33:1195–1203. https://doi.org/10.1016/j.ijhydene.2007.12.011
Don MM, Shoparwe NF (2010) Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochem Eng J 49:95–103. https://doi.org/10.1016/j.bej.2009.12.001
Luedeking R, Piret EL (1959) A kinetic study of the lactic acid fermentation. Batch process at controlled pH. J Biochem Microbiol Technol Eng 1:393–412. https://doi.org/10.1002/jbmte.390010406
Sangyoka S, Reungsang A, Lin C-Y (2016) Optimization of biohydrogen production from sugarcane bagasse by mixed cultures using a statistical method. Sustain Environ Res 26:235–242. https://doi.org/10.1016/j.serj.2016.05.001
Saraphirom P, Reungsang A (2010) Optimization of biohydrogen production from sweet sorghum syrup using statistical methods. Int J Hydrog Energy 35:13435–13444. https://doi.org/10.1016/j.ijhydene.2009.11.122
Lu C, Zhang Z, Zhou X, Hu J, Ge X, Xia C, Zhao J, Wang Y, Jing Y, Li Y (2018) Effect of substrate concentration on hydrogen production by photo-fermentation in the pilot-scale baffled bioreactor. Bioresour Technol 247:1173–1176. https://doi.org/10.1016/j.biortech.2017.07.122
Ghosh D, Sobro IF, Hallenbeck PC (2012) Optimization of the hydrogen yield from single-stage photofermentation of glucose by Rhodobacter capsulatus JP91 using response surface methodology. Bioresour Technol 123:199–206. https://doi.org/10.1016/j.biortech.2012.07.061
Hitit ZY, Lazaro CZ, Hallenbeck PC (2017) Hydrogen production by co-cultures of Clostridium butyricum and Rhodospeudomonas palustris: optimization of yield using response surface methodology. Int J Hydrog Energy 42:6578–6589. https://doi.org/10.1016/j.ijhydene.2016.12.122
Sun Q, Xiao W, Xi D, Shi J, Yan X, Zhou Z (2010) Statistical optimization of biohydrogen production from sucrose by a co-culture of Clostridium acidisoli and Rhodobacter sphaeroides. Int J Hydrog Energy 35:4076–4084. https://doi.org/10.1016/j.ijhydene.2010.01.145
Hassan N, Jalil A, Vo D, Nabgan W (2020) An overview on the efficiency of biohydrogen production from cellulose. Biomass Convers Biorefin:1–23. https://doi.org/10.1007/s13399-020-01125-x
Chen C-Y, Lu W-B, Wu J-F, Chang J-S (2007) Enhancing phototrophic hydrogen production of Rhodopseudomonas palustris via statistical experimental design. Int J Hydrog Energy 32:940–949. https://doi.org/10.1016/j.ijhydene.2006.09.021
Dasgupta CN, Gilbert JJ, Lindblad P, Heidorn T, Borgvang SA, Skjanes K, Das D (2010) Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int J Hydrog Energy 35:10218–10238. https://doi.org/10.1016/j.ijhydene.2010.06.029
Tiang MF, Hanipa MAF, Abdul PM, Jahim JM, Mahmod SS, Takriff MS, Lay C-H, Reungsang A, Wu S-Y (2020) Recent advanced biotechnological strategies to enhance photo-fermentative biohydrogen production by purple non-sulphur bacteria: an overview. Int J Hydrog Energy:13211–13230. https://doi.org/10.1016/j.ijhydene.2020.03.033
Garimella S, Vimal A, Merugu R, Kumar A (2019) Optimization for enhanced hydrogen production from Rhodobacter sphaeroides using response surface methodology. SN Appl Sci 1:156
Mishra P, Ab Wahid Z, Zaid RM, Rana S, Tabassum S, Karim A, Singh L, Islam MA, Jaing X, Sakinah M (2020) Kinetics and statistical optimization study of bio-hydrogen production using the immobilized photo-bacterium. Biomass Convers Biorefin:1–12. https://doi.org/10.1007/s13399-020-00835-6
Özgen C (2011) Microarray analysis of the effects of heat and cold stress on hydrogen production metabolism of Rhodobacter capsulatus. Citeseer
Phadtare S (2004) Recent developments in bacterial cold-shock response. Curr Issues Mol Biol 6:125–136
Chen W-H, Chen S-Y, Khanal SK, Sung S (2006) Kinetic study of biological hydrogen production by anaerobic fermentation. Int J Hydrog Energy 31:2170–2178. https://doi.org/10.1016/j.ijhydene.2006.02.020
Chen C-Y, Yeh K-L, Lo Y-C, Wang H-M, Chang J-S (2010) Engineering strategies for the enhanced photo-H2 production using effluents of dark fermentation processes as substrate. Int J Hydrog Energy 35:13356–13364. https://doi.org/10.1016/j.ijhydene.2009.11.070
Han H, Liu B, Yang H, Shen J (2012) Effect of carbon sources on the photobiological production of hydrogen using Rhodobacter sphaeroides RV. Int J Hydrog Energy 37:12167–12174. https://doi.org/10.1016/j.ijhydene.2012.03.134
Mullai P, Rene ER (2013) Sridevi K (2013) Biohydrogen production and kinetic modeling using sediment microorganisms of pichavaram mangroves, India. Biomed Res Int 2013:1–9. https://doi.org/10.1155/2013/265618
Gadhe A, Sonawane SS, Varma MN (2014) Kinetic analysis of biohydrogen production from complex dairy wastewater under optimized condition. Int J Hydrog Energy 39:1306–1314
Hu B, Li Y, Zhu S, Zhang H, Jing Y, Jiang D, He C, Zhang Z (2020) Evaluation of biohydrogen yield potential and electron balance in the photo-fermentation process with different initial pH from starch agricultural leftover. Bioresour Technol:122900. https://doi.org/10.1016/j.biortech.2020.122900
Shi XY, Yu HQ (2005) Optimization of glutamate concentration and pH for H2 production from volatile fatty acids by Rhodopseudomonas capsulata. Lett Appl Microbiol 40:401–406. https://doi.org/10.1111/j.1472-765X.2005.01700.x
Uyar B, Eroglu I, Yücel M, Gündüz U, Türker L (2007) Effect of light intensity, wavelength and illumination protocol on hydrogen production in photobioreactors. Int J Hydrog Energy 32:4670–4677. https://doi.org/10.1016/j.ijhydene.2007.07.002
Basak N, Das D (2009) Photofermentative hydrogen production using purple non-sulfur bacteria Rhodobacter sphaeroides OU 001 in an annular photobioreactor: a case study. Biomass Bioenergy 33:911–919. https://doi.org/10.1016/j.biombioe.2009.02.007
Adessi A, McKinlay JB, Harwood CS, De Philippis R (2012) A Rhodopseudomonas palustris nifA* mutant produces H2 from NH4+-containing vegetable wastes. Int J Hydrog Energy 37:15893–15900
Zagrodnik R, Thiel M, Seifert K, Włodarczak M, Łaniecki M (2013) Application of immobilized Rhodobacter sphaeroides bacteria in hydrogen generation process under semi-continuous conditions. Int J Hydrog Energy 38:7632–7639. https://doi.org/10.1016/j.ijhydene.2010.07.015
Cardena R, Moreno G, Valdez-Vazquez I, Buitrón G (2015) Optimization of volatile fatty acids concentration for photofermentative hydrogen production by a consortium. Int J Hydrog Energy 40:17212–17223. https://doi.org/10.1016/j.ijhydene.2015.10.020
Corneli E, Adessi A, Dragoni F, Ragaglini G, Bonari E, De Philippis R (2016) Agroindustrial residues and energy crops for the production of hydrogen and poly-β-hydroxybutyrate via photofermentation. Bioresour Technol 216:941–947. https://doi.org/10.1016/j.biortech.2016.06.046
Silva FTM, Moreira LR, de Souza FJ, Batista FRX, Cardoso VL (2016) Replacement of sugars to hydrogen production by Rhodobacter capsulatus using dark fermentation effluent as substrate. Bioresour Technol 200:72–80. https://doi.org/10.1016/j.biortech.2015.10.002
Krujatz F, Härtel P, Helbig K, Haufe N, Thierfelder S, Bley T, Weber J (2015) Hydrogen production by Rhodobacter sphaeroides DSM 158 under intense irradiation. Bioresour Technol 175:82–90. https://doi.org/10.1016/j.biortech.2014.10.061
Li X, Dai Z-Z, Wang Y-H, Zhang S-L (2011) Enhancement of phototrophic hydrogen production by Rhodobacter sphaeroides ZX-5 using fed-batch operation based on ORP level. Int J Hydrog Energy 36:12794–12802. https://doi.org/10.1016/j.ijhydene.2011.07.070
Acknowledgements
The first author, Raman Rao, wishes to thank the Ministry of Human Resource & Development, under the aegis of the Government of India for providing the fellowship. The authors are indebted to the Dr. B R Ambedkar National Institute of Technology Jalandhar (India) for providing continuous support and facilities to carry out the present research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RR: visualization, investigation, data curation, software, formal analysis, writing original draft. NB: conceptualization, project administration, methodology, supervision, investigation, and writing—review and editing.
Corresponding author
Ethics declarations
Research involving human participants and/or animals
No
Conflict of interest
The authors declare no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Batch hydrogen production from dark fermentative cheese whey effluent in 2-L double-walled cylindrical photobioreactor;
• Total organic acid concentration, temperature, and light intensity affect hydrogen production rate;
• Fermentation experiment was designed using Box-Behnken design of RSM;
• Optimal hydrogen production rate was 41.65 mL L-1 h-1 at total organic acid concentration of 12 g L-1, temperature 31 °C, and light intensity 10 klx;
• Modelling of photo fermentation by unstructured models at statistically optimized variables gave satisfactory fitting with experimental data;
• Statistical and mathematical modelling of biohydrogen production may provide useful insights for waste to hydrogen endeavors.
Supplementary Information
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Rao, R., Basak, N. Process optimization and mathematical modelling of photo-fermentative hydrogen production from dark fermentative cheese whey effluent by Rhodobacter sphaeroides O.U.001 in 2-L cylindrical bioreactor. Biomass Conv. Bioref. 13, 3929–3952 (2023). https://doi.org/10.1007/s13399-021-01377-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01377-1