Abstract
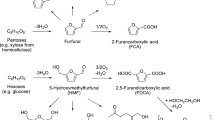
In this work, biomass-derived furfural has been selectively oxidized to 2(5H)-furanone using aqueous hydrogen peroxide as the green oxidant. Among various homogeneous acid catalysts screened for the transformation, trifluoroacetic acid (TFA) was found to be the most suitable candidate that afforded up to 52% isolated yield of 2(5H)-furanone under mild conditions (RT, 1 h). In addition, succinic acid was recovered in nearly 20% yield from the aqueous layer. The organic solvent-free, gram-scale reaction was optimized on temperature, the molar ratio of H2O2 and furfural, and the amount of TFA used.

Grapical abstract



Similar content being viewed by others
References
Florentino G, Ripa M, Ulgiati S (2017) Chemicals from biomass: technological versus environmental feasibility. A review. Biofuels Bioprod Biorefin 11:195–214. https://doi.org/10.1002/bbb.1729
Wu L, Moteki T, Gokhale AA, Flaherty DW, Toste FD (2016) Production of fuels and chemicals from biomass: condensation reactions and beyond. Chem 1:32–58. https://doi.org/10.1016/j.chempr.2016.05.002
Serrano-Ruiz JC, West RM, Dumesic JA (2010) Catalytic conversion of renewable biomass resources to fuels and chemicals. Annu Rev Chem Biomol Eng 1:79–100. https://doi.org/10.1146/annurev-chembioeng-073009-100935
Mariscal R, Maireles-Torres P, Ojeda M, Sadaba I, Lopez Granados M (2016) Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9:1144–1189. https://doi.org/10.1039/C5EE02666K
Li X, Jia P, Wang T (2016) Furfural: a promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal 6:7621–7640. https://doi.org/10.1021/acscatal.6b01838
Gupta NK, Fukuoka A, Nakajima K (2018) Metal-free and selective oxidation of furfural to furoic acid with an N-heterocyclic carbene catalyst. ACS Sustain Chem Eng 6:3434–3442. https://doi.org/10.1021/acssuschemeng.7b03681
Bechthold I, Bretz K, Kabasci S, Kopitzky R, Springer A (2008) Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol 31:647–654. https://doi.org/10.1002/ceat.200800063
Wojcieszak R, Santarelli F, Paul S, Dumeignil F, Cavani F, Gonçalves RV (2015) Recent developments in maleic acid synthesis from bio-based chemicals. Sustainable Chem Processes 3:9. https://doi.org/10.1186/s40508-015-0034-5
The SciFinder search on 2(5H)-furanone and succinic acid refined by ‘biomass’ as the research topic displayed 318 and 2391 literature references, respectively
Shiramizu M, Toste DF (2013) Expanding the scope of biomass-derived chemicals through tandem reactions based on oxorhenium-catalyzed deoxydehydration. Angew Chem Int Ed 52:12904–12909. https://doi.org/10.1002/anie.201307564
Li X, Li Y, Wang T (2019) Effect of oxide supports on Pt-Ni bimetallic catalysts for the selective hydrogenation of biomass-derived 2(5H)-furanone. Catal Today 319:93–99. https://doi.org/10.1016/j.cattod.2018.03.053
Song Q, Zhao J, Zhang G, Peruch F, Carlotti S (2020) Ring-opening (co)polymerization of γ-butyrolactone: a review. Polymer J 52:3–11. https://doi.org/10.1038/s41428-019-0265-5
Rossi R, Lessi M, Manzini C, Marianetti G, Bellina F (2017) Synthesis and biological properties of 2(5H)-furanones featuring bromine atoms on the heterocyclic ring and/or brominated substituents. Curr Org Chem 21:964–1018. https://doi.org/10.2174/1385272821666170111151917
I. Effenberger, T. Hoffmann, R. Jonczyk, W. Schwab, Novel biotechnological glucosylation of high-impact aroma chemicals, 3(2H)-and 2(5H)-furanones. Sci Rep 10943 (2019). https://doi.org/10.1038/s41598-019-47514-9
Lima CGS, Monteiro JL, De Melo Lima T, Paixao MW, Correa AG (2018) Angelica lactones: from biomass-derived platform chemicals to value-added products. ChemSusChem 11:25–47. https://doi.org/10.1002/cssc.201701469
Gassama A, Ernenwein C, Hoffmann N (2010) Synthesis of surfactants from furfural derived 2[5H]-furanone and fatty amines. Green Chem 12:859–865. https://doi.org/10.1039/B924187F
Yan J, Wang H, Yang Z, He Y (2019) An efficient catalytic sulfonyloxylactonization of alkenoic acids using hypervalent iodine(III) reagent. Syn Lett 16:2669–2672. https://doi.org/10.1055/s-0029-1217977
Xie X, Stahl SS (2015) Efficient and selective cu/nitroxyl-catalyzed methods for aerobic oxidative lactonization of diols. J Am Chem Soc 137:3767–3770. https://doi.org/10.1021/jacs.5b01036
Chen Y-Z, Wu L-Z, Peng M-L, Zhang M-L, Zhang D, Zhang L-P, Tung C-H (2016) Synthesis of α, β-unsaturated γ-lactones via photooxygenation of 2, 3-dihydrofurans followed by ferrous ion-catalyzed gem-dehydration. Tetrahedron 62:10688–10693. https://doi.org/10.1016/j.tet.2006.08.085
Teong SP, Li X, Zhang Y (2019) Hydrogen peroxide as an oxidant in biomass-to-chemical processes of industrial interest. Green Chem 21:5753–5780. https://doi.org/10.1039/C9GC02445J
Araji N, Madjinza DD, Chatel G, Moores A, Jerome F, Vigier KDO (2017) Synthesis of maleic and fumaric acids from furfural in the presence of betaine hydrochloride and hydrogen peroxide. Green Chem 19:98–101. https://doi.org/10.1039/C6GC02620F
Teong SP, Li X, Zhang Y (2019) Hydrogen peroxide as oxidant in biomass-to-chemical processes with industrial interest. Green Chem 21:5753–5780. https://doi.org/10.1039/c9gc02445j
Xiang X, Zhang B, Ding G, Cui J, Zheng H, Zhu Y (2016) The effect of Mg(OH)2 on furfural oxidation with H2O2. Catal Comm 86:41–45. https://doi.org/10.1016/j.catcom.2016.08.013
Li X, Lan X, Wang T (2016) Highly selective catalytic conversion of furfural to γ-butyrolactone. Green Chem 18:638–642. https://doi.org/10.1039/C5GC02411K
Li X, Lan X, Wang T (2016) Selective oxidation of furfural in a bi-phasic system with homogeneous acid catalyst. Catal Today 276:97–104. https://doi.org/10.1016/j.cattod.2015.11.036
Kortet S, Claraz A, Pihko PM (2020) Catalytic enantioselective total synthesis of (+)-lycoperdic acid. Org Lett 22:3010–3013. https://doi.org/10.1021/acs.orglett.0c00772
Khotavivattana T, Khamkhenshorngphanuch T, Rassamee K, Siripong P, Vilaivan T (2018) Diverted total synthesis of melodorinol analogues and evaluation of their cytotoxicity. Tetrahedron Lett 59:2711–2715. https://doi.org/10.1016/j.tetlet.2018.06.005
Song L, Yao H, Zhu L, Tong R (2013) Asymmetric total syntheses of (−)-penicipyrone and (−)-tenuipyrone via biomimetic cascade intermolecular Michael addition/cycloketalization. Org Lett 15:6–9. https://doi.org/10.1021/ol303071t
Shi Y-H, Wang Z, Shi Y, Deng W-P (2012) Facile and highly diastereoselective synthesis of 3-aminooxindoles via AgOAc-catalyzed vinylogous Mannich reaction. Tetrahedron 68:3649–3653. https://doi.org/10.1016/j.tet.2012.02.046
Akiya N, Savage PE (1988) Role of water in formic acid decomposition. AICHE J 44:405–415. https://doi.org/10.1002/aic.690440217
Yasaka Y, Yoshida K, Wakai C, Matubayasi N, Nakahara M (2006) Kinetic and equilibrium study on formic acid decomposition in relation to the water-gas-shift reaction. J Phy Chem A 110:11082–11090. https://doi.org/10.1021/jp0626768
Peng X, Chen L, Yan Z (2018) Sulfonic polymer catalysts for converting of furfural to high-value chemicals. Energy Sources, Part A 40:2342–2353. https://doi.org/10.1080/15567036.2018.1488018
Zvarych V, Nakonechna A, Marchenko M, Khudyi O, Lubenets V, Khuda L, Kushniryk O, Novikov V (2019) Hydrogen peroxide oxygenation of furan-2-carbaldehyde via an easy, green method. J Argic Food Chem 67:3114–3117. https://doi.org/10.1021/acs.jafc.8b06284
Lopez SE, Salazar J (2013) Trifluoroacetic acid: uses and recent applications in organic synthesis. J Fluor Chem 156:73–100. https://doi.org/10.1016/j.jfluchem.2013.09.004
Venier CG, Squires TG, Chen Y-Y, Hussmann GP, Shei JC, Smith BF (1982) Peroxytrifluoroacetic acid. A convenient reagent for the preparation of sulfoxides and sulfones. J Org Chem 47:3773–3774. https://doi.org/10.1021/jo00140a040
A.D. Natu, A.S. Burde, R.A. Limaye, M.V. Paradkar, Acceleration of the Dakin reaction by trifluoroacetic acid. J Chem Res 38 (2014) 381–382. https://doi.org/10.3184/174751914X14014814873316
Dutta S, Wu L, Mascal M (2015) Efficient, metal-free production of succinic acid by oxidation of biomass-derived levulinic acid with hydrogen peroxide. Green Chem 17:2335–2338. https://doi.org/10.1039/C5GC00098J
Nasman J-AH, Pensar KG (1985) An improved one-pot preparation of 2-furanones. Synthesis 8:786–788. https://doi.org/10.1055/s-1985-31349 (The 1H & 13C-NMR spectral data of 2(5H)-furanone, 1 match with those in the literature)
Tripathi NK, Kishor Mishra B, Tiwari A, Choubey ON (2016) Trifluoroacetic acid recovery from industrial aqueous effluent. IORS J Appl Chem 9:70–72. https://doi.org/10.9790/5736-0911017072
Mahajan YS, Shah AK, Kamath RS, Salve NB, Mahajani SM (2008) Recovery of trifluoroacetic acid from dilute aqueous solutions by reactive distillation. Sep Purif Technol 59:58–66. https://doi.org/10.1016/j.seppur.2007.05.027
Acknowledgments
The authors want to thank Mangalore University, Karnataka, and TIFR, Hyderabad, for helping in the NMR and HPLC data collection.
Funding
This study was financially supported by the Council of Scientific and Industrial Research (CSIR), India, under Scheme 02(0301)/17/EMR-II.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
The 1H-NMR spectrum of the crude reaction mixture, NMR spectra (1H, 13C) of the isolated products (i.e., 2-furanone and succinic acid), and HPLC data of the aqueous reaction mixture (before and after extraction of 2-furanone) are provided in the supporting information. (PDF 363 kb)
Rights and permissions
About this article
Cite this article
Bhat, N.S., Kumar, R., Jana, A. et al. Selective oxidation of biomass-derived furfural to 2(5H)-furanone using trifluoroacetic acid as the catalyst and hydrogen peroxide as a green oxidant. Biomass Conv. Bioref. 13, 1029–1034 (2023). https://doi.org/10.1007/s13399-021-01297-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01297-0