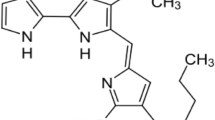
Abstract
Increasing utilization of artificial chemical dyes as coloring agent and their associated detrimental effects on human health have instigated the search for novel natural pigments for applications in the food and pharmaceutical industries. A wide range of advancements have been recorded for utilization of naturally extracted pigments, owing to their assurance in affirmative health attentiveness in the past few decades. Therefore, it is essential to investigate various potential natural resources such as plants and microbes from where food-grade colorants can be obtained. Several drawbacks such as low water solubility, instability to light, heat, or adverse pH are associated with natural pigments extracted from plants. On the contrary, microbial pigments having higher stability and productivity are considered a potential alternative to synthetic dyes. Prodigiosin is a linear tripyrrole natural red pigment obtained from Serratia marcescens which is produced as a secondary metabolite. Prodigiosin and their derivatives act as proapoptotic agents against diverse cancer cell lines and also play a vital role in numerous cellular targets, including multi-drug resistance cells with reduced or no reported adverse side effects. Owing to its high potential in various sectors, there is an urgent need to explore and exploit the biosynthesis of prodigiosin using various biotechnological approaches. Furthermore, multifarious aspects in commercial-scale production of prodigiosin along with its contemporary utilization have been critically assessed in this article.



Similar content being viewed by others
Abbreviations
- PG:
-
Prodigiosin or prodiginines.
- MBC:
-
4-Methoxy-2-2′-bipyrrole-5-carbaldehyde
- MPP:
-
2-Methyl-3-pentylpyrrole
- HBC:
-
4-Hydroxyl-2,2′-bipyrrole-5-carbaldehyde
- HBM:
-
4-Hydroxy-2,2′-bipyrrole-5-methanol
- Pig:
-
Pigment
- UV:
-
Ultraviolet
- HCl:
-
Hydrogen chloride
- Pig clusters:
-
(pigN, pigA, pigF, pigM, pigE, pigJ, pigH, pigD, pigB, pigBC)-two
- Red H:
-
Undecylprodigiosin
- Hap C:
-
Prodigiosin
- Rph H:
-
Prodigiosin R1
- Tam Q:
-
Tambjamine YPI
- YP 1:
-
Pseudoalteromonas tunicate
- Tam:
-
Tambjamine producer
- Rph:
-
Roseophilin producer
- LD50 :
-
Lethal dose 50%
- DMSO:
-
Dimethyl sulfoxide
- QSAR:
-
Quantitative structure-activity relationship
- TRAIL:
-
TNF-related apoptosis inducing ligand
- CP-MLR:
-
Combinatorial protocol in multiple linear regression
- Caspase:
-
Cysteine-aspartate proteases family
- Bcl-2:
-
B cell lymphoma 2
- Fas:
-
FS-7 associated surface antigen
- TLC:
-
Thin layer chromatography
- TNF:
-
Tumor necrosis factor
- HPLC:
-
High-performance liquid chromatography
- NMR:
-
Nuclear magnetic resonance
- GC-MS:
-
Gas chromatography mass spectrophotometer
- MS:
-
Mass spectrophotometer
- FTIR:
-
Fourier-transform infrared spectrophotometer
- DCW:
-
Dry cell weight
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ABTS:
-
2,2′-Azino-bis 3-ethylbenzthiazoline-6-sulfonic acid
- YPI:
-
Pseudoalteromonas tunicata
- REDL:
-
RED Q, AFA, etc.
- DNA:
-
Di-oxy ribonucleic acid
- TAM H:
-
Tambjamine H
- TAM Q:
-
Tambjamine Q
- RNA:
-
Ribonucleic acid
- ABTS:
-
2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
- GvHD:
-
Graft versus host disease
- IUPAC:
-
International Union of Pure and Applied Chemistry
- LC-HRMS:
-
Liquid chromatography-high-resolution mass spectrometry
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- ATP:
-
Adenosine triphosphate
- CP-MLR:
-
Combinatorial protocol in multiple linear regressions
- IPTG:
-
Isopropyl-β-d-thiogalactoside
- HEp-2:
-
Laryngeal epidermoid carcinoma
- HL-60:
-
Human promyelocytic leukemia
- NCIH-292:
-
Lung mucoepidermoid carcinoma
- MCF-7:
-
Breast adenocarcinoma cell lines
- HepG2:
-
The human liver cancer cell
- A549:
-
Human lung carcinoma
- K562:
-
Human bone marrow chronic
- B-CLL:
-
B cell chronic lymphocytic leukemia
- U937:
-
Human Caucasian histiocytic lymphoma derived from malignant cells
- IMR-32:
-
A human neuroblastoma cell line
- MCF-7:
-
MCF-7 is a breast cancer cell line isolated in 1970 from a 69-year-old Caucasian woman
- DLD-1:
-
One of four colorectal adenocarcinoma cell
- SW-620:
-
SW-620 cell line expresses carcinoembryonic antigen
- P27 :
-
Cyclin-dependent kinase inhibitor 1B (p27Kip1) is an enzyme inhibitor that in humans is encoded by the CDKN1B gene
- P53 :
-
p53, also known as TP53 or tumour protein (EC: 2.7. 1.37) is a gene that codes for a protein that regulates the cell cycle and hence functions as a tumour suppression
- P73 :
-
p73 (encoded by TP73 gene) is a p53-related protein that functions as a transcriptional factor
- P38 :
-
P38 mitogen–activated protein kinases
- P38 MAP Kinase (MAPK):
-
also called RK or CSBP (cytokinin-specific binding protein), is the mammalian orthologue of the yeast Hog1p MAP kinase, which participates in a signaling cascade controlling cellular responses to cytokines and stress
- Cdk 2:
-
Cyclin-dependent kinase 2
- PKC:
-
Protein kinase C
- MAPK:
-
Mitogen-activated protein kinase
- PTP1B:
-
Protein-tyrosine phosphatase 1B
- PP2A:
-
Protein phosphatase 2
- JNK:
-
Jun N-terminal kinase
- RAD 51:
-
RAD51 gene provides instructions for making a protein that is essential for repairing damaged DNA
- c-Jun:
-
It is a protein that in humans is encoded by the JUN gene
- ∆Np73:
-
∆Np73-α synergizes with specificity protein (Sp1)
- mTORC1/2:
-
Mammalian target of rapamycin
- PI3K-p85:
-
Phosphoinositide 3-kinases
- SKP2:
-
S-Phase kinase-associated protein 2
- PKB:
-
Protein kinase B
- HL60:
-
Human acute promyelocytic leukemia
- Hep G2:
-
Human hepatocellular carcinoma
- HCT 116:
-
Human colorectal carcinoma
- H23-SK-MEL-5:
-
SK-MEL-5 is one of a series of melanoma cell lines established from patient-derived tumor samples
References
Darshan N, Manonmani H (2015) Prodigiosin and its potential applications. J Food Sci Technol 52(9):5393–5407. https://doi.org/10.1007/s13197-015-1740-4
Sen T, Barrow CJ, Deshmukh SK (2019) Microbial pigments in the food industry—challenges and the way forward. Front Nutr 6. https://doi.org/10.3389/fnut.2019.00007
Dilrukshi PT, Munasinghe H, Silva ABG, De Silva PGSM (2019) Identification of synthetic food colours in selected confectioneries and beverages in Jaffna District, Sri Lanka. J Food Qual 2019. https://doi.org/10.1155/2019/7453169
Panesar R, Kaur S, Panesar PS (2015) Production of microbial pigments utilizing agro-industrial waste: a review. Curr Opin Food Sci 1:70–76. https://doi.org/10.1016/j.cofs.2014.12.002
Sánchez-Contreras A, Jiménez M, Sanchez S (2000) Bioconversion of lutein to products with aroma. Appl Microbiol Biotechnol 54(4):528–534. https://doi.org/10.1007/s002530000403
Lin S-R, Chen Y-H, Tseng F-J, Weng C-F (2020) The production and bioactivity of prodigiosin: quo vadis? Drug Discov Today. https://doi.org/10.1016/j.drudis.2020.03.017
Venil CK, Aruldass CA, Dufossé L, Zakaria ZA, Ahmad WA (2014) Current perspective on bacterial pigments: emerging sustainable compounds with coloring and biological properties for the industry–an incisive evaluation. RSC Adv 4(74):39523–39529. https://doi.org/10.1039/C4RA06162D
Abdullah N, Sekak KA, Ahmad M, Effendi TB (2014) Characteristics of electrospun PVA-Aloe vera nanofibres produced via electrospinning. In: Proceedings of the international colloquium in textile engineering, fashion, apparel and design 2014 (ICTEFAD 2014). Springer, pp 7–11. https://doi.org/10.1007/978-981-287-011-7
Nagpal N, Munjal N, Chatterjee S (2011) Microbial pigments with health benefits-a mini review. Trends Biosci 4(2):157–160. https://doi.org/10.1007/s13205-018-1227-x
Sakai-Kawada FE, Ip CG, Hagiwara KA, Awaya JD (2019) Biosynthesis and bioactivity of prodiginine analogues in marine bacteria, Pseudoalteromonas: a mini review. Front Microbiol 10:1715. https://doi.org/10.3389/fmicb.2019.01715
Casullo de Araújo HW, Fukushima K, Takaki GMC (2010) Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 15(10):6931–6940. https://doi.org/10.3390/molecules15106931
Stankovic N, Radulovic V, Petkovic M, Vuckovic I, Jadranin M, Vasiljevic B, Nikodinovic-Runic J (2012) Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Appl Microbiol Biotechnol 96(5):1217–1231. https://doi.org/10.1007/s00253-012-4237-3
Harris AK, Williamson NR, Slater H, Cox A, Abbasi S, Foulds I, Simonsen HT, Leeper FJ, Salmond GP (2004) The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species-and strain-dependent genome context variation. Microbiology 150(11):3547–3560. https://doi.org/10.1099/mic.0.27222-0
Hu DX, Withall DM, Challis GL, Thomson RJ (2016) Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem Rev 116(14):7818–7853. https://doi.org/10.1021/acs.chemrev.6b00024
Setiyono E, Adhiwibawa MAS, Indrawati R, Prihastyanti MNU, Shioi Y, Brotosudarmo THP (2020) An Indonesian marine bacterium, Pseudoalteromonas rubra, produces antimicrobial prodiginine pigments. ACS Omega 5(9):4626–4635. https://doi.org/10.1021/acsomega.9b04322
de Rond T, Stow P, Eigl I, Johnson RE, Chan LJG, Goyal G, Baidoo EE, Hillson NJ, Petzold CJ, Sarpong R (2017) Oxidative cyclization of prodigiosin by an alkylglycerol monooxygenase-like enzyme. Nat Chem Biol 13(11):1155. https://doi.org/10.1038/nchembio.2471
Withall DM, Haynes SW, Challis GL (2015) Stereochemistry and mechanism of undecylprodigiosin oxidative carbocyclization to streptorubin B by the rieske oxygenase RedG. J Am Chem Soc 137(24):7889–7897. https://doi.org/10.1021/jacs.5b03994
Kimata S, Izawa M, Kawasaki T, Hayakawa Y (2017) Identification of a prodigiosin cyclization gene in the roseophilin producer and production of a new cyclized prodigiosin in a heterologous host. J Antibiot 70(2):196–199. https://doi.org/10.1038/ja.2016.94
Vitale GA, Sciarretta M, Palma Esposito F, January GG, Giaccio M, Bunk B, Spröer C, Bajerski F, Power D, Festa C (2020) Genomics–metabolomics profiling disclosed marine vibrio spartinae 3.6 as a producer of a new branched side chain prodigiosin. J Nat Prod 83(5):1495–1504. https://doi.org/10.1021/acs.jnatprod.9b01159
Sharma R, Rao MR, Ravikanth M (2017) α-Pyrrolyl dipyrrins as suitable ligands for coordination chemistry. Coord Chem Rev 348:92–120. https://doi.org/10.1016/j.ccr.2017.08.002
Manas NHA, Yee CL, Tesfamariam YM, Zulkharnain A, Mahmud H, Mahmod DSA, Fuzi SFZM, Azelee NIW (2020) Effects of oil substrate supplementation on production of prodigiosin by Serratia nematodiphila for dye sensitized solar cell. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2020.04.011
Darshan N, Manonmani H (2016) Prodigiosin inhibits motility and activates bacterial cell death revealing molecular biomarkers of programmed cell death. AMB Express 6(1):50. https://doi.org/10.1186/s13568-016-0222-z
Jehlička J, Němec I, Varnali T, Culka A, Svatoš A, Frank O, Oren A, Edwards HG (2016) The pink pigment prodigiosin: vibrational spectroscopy and DFT calculations. Dyes Pigments 134:234–243. https://doi.org/10.1016/j.dyepig.2016.07.018
Ramesh C, Vinithkumar NV, Kirubagaran R, Venil CK, Dufossé L (2020) Applications of prodigiosin extracted from marine red pigmented bacteria Zooshikella sp. and Actinomycete streptomyces sp. Microorganisms 8(4):556. https://doi.org/10.3390/microorganisms8040556
Suryawanshi RK, Patil CD, Borase HP, Salunke BK, Patil SV (2014) Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl Biochem Biotechnol 173(5):1209–1221. https://doi.org/10.1007/s12010-014-0921-3
Sumathi C, MohanaPriya D, Swarnalatha S, Dinesh MG, Sekaran G (2014) Production of prodigiosin using tannery fleshing and evaluating its pharmacological effects. Sci World J 2014:290327. https://doi.org/10.1155/2014/290327
Cerdeño AM, Bibb MJ, Challis GL (2001) Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3 (2): new mechanisms for chain initiation and termination in modular multienzymes. Chem Biol 8(8):817–829. https://doi.org/10.1016/s1074-5521(01)00054-0
Kim D, Lee JS, Park Y, Kim JF, Jeong H, Oh TK, Kim BS, Lee CH (2007) Biosynthesis of antibiotic prodiginines in the marine bacterium Hahella chejuensis KCTC 2396. J Appl Microbiol 102(4):937–944. https://doi.org/10.1111/j.1365-2672.2006.03172.x
Burke C, Thomas T, Egan S, Kjelleberg S (2007) The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ Microbiol 9(3):814–818. https://doi.org/10.1111/j.1462-2920.2006.01177.x
Kawasaki T, Sakurai F, S-y N, Hayakawa Y (2009) Prodigiosin biosynthesis gene cluster in the roseophilin producer Streptomyces griseoviridis. J Antibiot 62(5):271–276. https://doi.org/10.1038/ja.2009.27
Williamson NR, Simonsen HT, Ahmed RA, Goldet G, Slater H, Woodley L, Leeper FJ, Salmond GP (2005) Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol Microbiol 56(4):971–989. https://doi.org/10.1111/j.1365-2958.2005.04602.x)
Jung H-J, Ho J-K (2006) Chemical genomics with natural products. J Microbiol Biotechnol 16(5):651–660. https://doi.org/10.1016/s1074-5521(02)00100-x
Stanley AE, Walton LJ, Zerikly MK, Corre C, Challis GL (2006) Elucidation of the Streptomyces coelicolor pathway to 4-methoxy-2, 2′-bipyrrole-5-carboxaldehyde, an intermediate in prodiginine biosynthesis. Chem Commun 38:3981–3983. https://doi.org/10.1039/b609556a
Mo S, Sydor PK, Corre C, Alhamadsheh MM, Stanley AE, Haynes SW, Song L, Reynolds KA, Challis GL (2008) Elucidation of the Streptomyces coelicolor pathway to 2-undecylpyrrole, a key intermediate in undecylprodiginine and streptorubin B biosynthesis. Chem Biol 15(2):137–148. https://doi.org/10.1016/j.chembiol.2007.11.015
Schloss PD, Allen HK, Klimowicz AK, Mlot C, Gross JA, Savengsuksa S, McEllin J, Clardy J, Ruess RW, Handelsman J (2010) Psychrotrophic strain of Janthinobacterium lividum from a cold Alaskan soil produces prodigiosin. DNA Cell Biol 29(9):533–541. https://doi.org/10.1089/dna.2010.1020
Domröse A, Klein A, Hage-Hülsmann J, Thies S, Svensson V, Classen TPJ, Jaeger JE, Drepper T, Loeschcke A (2015) Efficient recombinant production of prodigiosin in Pseudomonas putida. Front Microbiol 6:972. https://doi.org/10.3389/fmicb.2015.00972
Chen W-C, Tsai M-J, Soo P-C, Wang L-F, Tsai S-L, Chang Y-K, Wei Y-H (2018) Construction and co-cultivation of two mutant strains harboring key precursor genes to produce prodigiosin. J Biosci Bioeng 126(6):783–789. https://doi.org/10.1016/j.jbiosc.2018.06.010
Kwon S-K, Park Y-K, Kim JF (2010) Genome-wide screening and identification of factors affecting the biosynthesis of prodigiosin by Hahella chejuensis, using Escherichia coli as a surrogate host. Appl Environ Microbiol 76(5):1661–1668. https://doi.org/10.1128/AEM.01468-09
Klein AS, Domröse A, Bongen P, Brass HU, Classen T, Loeschcke A, Drepper T, Laraia L, Sievers S, Jaeger K-E (2017) New prodigiosin derivatives obtained by mutasynthesis in Pseudomonas putida. ACS Synth Biol 6(9):1757–1765. https://doi.org/10.1021/acssynbio.7b00099
Klein AS, Brass HUC, Klebl DP, Classen T, Loeschcke A, Drepper T, Sievers S, Jaeger KE, Pietruszka J (2018) Preparation of cyclic prodiginines by mutasynthesis in Pseudomonas putida KT2440. ChemBioChem 19(14):1545–1552. https://doi.org/10.1002/cbic.201800154
You Z, Zhang S, Liu X, Wang Y (2018) Enhancement of prodigiosin synthetase (PigC) production from recombinant Escherichia coli through optimization of induction strategy and media. Prep Biochem Biotechnol 48(3):226–233. https://doi.org/10.1080/10826068.2017.1421965
Liu P, Zhu H, Zheng G, Jiang W, Lu Y (2017) Metabolic engineering of Streptomyces coelicolor for enhanced prodigiosins (RED) production. Sci China Life Sci 60(9):948–957. https://doi.org/10.1007/s11427-017-9117-x
Pan X, Sun C, Tang M, Liu C, Zhang J, You J, Osire T, Sun Y, Zhao Y, Xu M (2019) Loss of serine-type D-Ala-D-Ala carboxypeptidase DacA enhances prodigiosin production in Serratia marcescens. Front Bioeng Biotechnol 7:367. https://doi.org/10.3389/fbioe.2019.00367
Giri AV, Anandkumar N, Muthukumaran G, Pennathur G (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol 4(1):11. https://doi.org/10.1186/1471-2180-4-11
Wei Y-H, Chen W-C (2005) Enhanced production of prodigiosin-like pigment from Serratia marcescens SMΔR by medium improvement and oil-supplementation strategies. J Biosci Bioeng 99(6):616–622. https://doi.org/10.1263/jbb.99.616
Cang S, Sanada M, Johdo O, Ohta S, Nagamatsu Y, Yoshimoto A (2000) High production of prodigiosin by Serratia marcescens grown on ethanol. Biotechnol Lett 22(22):1761–1765. https://doi.org/10.1023/A:1005646102723
Siva R, Subha K, Bhakta D, Ghosh A, Babu S (2012) Characterization and enhanced production of prodigiosin from the spoiled coconut. Appl Biochem Biotechnol 166(1):187–196. https://doi.org/10.1007/s12010-011-9415-8
Hejazi A, Falkiner F (1997) Serratia marcescens. J Med Microbiol 46(11):903–912. https://doi.org/10.1099/00222615-46-11-903
Lin C, Jia X, Fang Y, Chen L, Zhang H, Lin R, Chen J (2019) Enhanced production of prodigiosin by Serratia marcescens FZSF02 in the form of pigment pellets. Electron J Biotechnol 40:58–64. https://doi.org/10.1016/j.ejbt.2019.04.007
Elkenawy NM, Yassin AS, Elhifnawy HN, Amin MA (2017) Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol Rep 14:47–53. https://doi.org/10.1016/j.btre.2017.04.001
J-l T, Wang X-d, Shen Y-l, D-z W (2005) Strategy for the improvement of prodigiosin production by a Serratia marcescens mutant through fed-batch fermentation. World J Microbiol Biotechnol 21(6-7):969–972. https://doi.org/10.1007/s11274-004-7257
Nguyen VB, Chen S-P, Nguyen TH, Nguyen MT, Tran TTT, Doan CT, Tran TN, Nguyen AD, Kuo Y-H, Wang S-L (2020) Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar Drugs 18(1):15. https://doi.org/10.3390/md18010015
Romanowski EG, LEHNER KM, Martin NC, Patel KR, Callaghan JD, Stella NA, Shanks RM (2019) Thermoregulation of prodigiosin biosynthesis by Serratia marcescens is controlled at the transcriptional level and requires HexS. Pol J Microbiol 68(1):43. https://doi.org/10.21307/pjm-2019-005
Haddix PL, Shanks RM (2018) Prodigiosin pigment of Serratia marcescens is associated with increased biomass production. Arch Microbiol 200(7):989–999. https://doi.org/10.1007/s00203-018-1508-0
Haddix PL, Shanks RM (2020) Production of prodigiosin pigment by Serratia marcescens is negatively associated with cellular ATP levels during high-rate, low cell density growth. Can J Microbiol. https://doi.org/10.1139/cjm-2019-0548
Guryanov I, Karamova N, Yusupova D, Gnezdilov O, Koshkarova L (2013) Bacterial pigment prodigiosin and its genotoxic effect. Russ J Bioorg Chem 39(1):106–111. https://doi.org/10.1134/S1068162012060040
Arivizhivendhan K, Mahesh M, Boopathy R, Sekaran G (2016) A novel method for the extraction of prodigiosin from bacterial fermenter integrated with sequential batch extraction reactor using magnetic iron oxide. Process Biochem 51(10):1731–1737. https://doi.org/10.1016/j.procbio.2016.07.012
Juang R-S, Yeh C-L (2014) Adsorptive recovery and purification of prodigiosin from methanol/water solutions of Serratia marcescens fermentation broth. Biotechnol Bioprocess Eng 19(1):159–168. https://doi.org/10.1007/s12257-013-0547-2
Juang R-S, Chen H-L, Lin Y-C (2012) Ultrafiltration of coagulation-pretreated Serratia marcescens fermentation broth: flux characteristics and prodigiosin recovery. Sep Sci Technol 47(13):1849–1856. https://doi.org/10.1080/01496395.2012.665117
Balasubramaniam B, Alexpandi R, Darjily DR (2019) Exploration of the optimized parameters for bioactive prodigiosin mass production and its biomedical applications in vitro as well as in silico. Biocatal Agric Biotechnol 22:101385. https://doi.org/10.1016/j.bcab.2019.101385
Yip C-H, Yarkoni O, Ajioka J, Wan K-L, Nathan S (2019) Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl Microbiol Biotechnol 103(4):1667–1680. https://doi.org/10.1007/s00253-018-09611-z
Arivizhivendhan K, Mahesh M, Boopathy R, Swarnalatha S, Mary RR, Sekaran G (2018) Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J Food Sci Technol 55(7):2661–2670. https://doi.org/10.1007/s13197-018-3188-9
Chen J, Li Y, Liu F, Hou D-X, Xu J, Zhao X, Yang F, Feng X (2019) Prodigiosin promotes Nrf2 activation to inhibit oxidative stress induced by microcystin-LR in HepG2 cells. Toxins 11(7):403. https://doi.org/10.3390/toxins11070403
Sajjad W, Ahmad S, Aziz I, Azam SS, Hasan F, Shah AA (2018) Antiproliferative, antioxidant and binding mechanism analysis of prodigiosin from newly isolated radio-resistant Streptomyces sp. strain WMA-LM31. Mol Biol Rep 45(6):1787–1798. https://doi.org/10.1007/s11033-018-4324-3
Lazović S, Leskovac A, Petrović S, Senerovic L, Krivokapić N, Mitrović T, Božović N, Vasić V, Nikodinovic-Runic J (2017) Biological effects of bacterial pigment undecylprodigiosin on human blood cells treated with atmospheric gas plasma in vitro. Exp Toxicol Pathol 69(1):55–62. https://doi.org/10.1016/j.etp.2016.11.003
Kalesperis G, Prahlad K, Lynch D (1975) Toxigenic studies with the antibiotic pigments from Serratia marcescens. Can J Microbiol 21(2):213–220. https://doi.org/10.1139/m75-030
Seah S-W, Nathan S, Wan K-L (2016) Toxicity evaluation of prodigiosin from Serratia marcescens in a Caenorhabditis elegans model. In: AIP Conference Proceedings, vol 1. AIP Publishing LLC, p 020015. https://doi.org/10.1063/1.4966725
Papireddy K, Smilkstein M, Kelly JX, Shweta SSM, Alhamadsheh M, Haynes SW, Challis GL, Reynolds KA (2011) Antimalarial activity of natural and synthetic prodiginines. J Med Chem 54(15):5296–5306. https://doi.org/10.1021/jm200543y
Rahul S, Chandrashekhar P, Hemant B, Bipinchandra S, Mouray E, Grellier P, Satish P (2015) In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol Int 64(5):353–356. https://doi.org/10.1016/j.parint.2015.05.004
You Z, Zhang S, Liu X, Zhang J, Wang Y, Peng Y, Wu W (2019) Insights into the anti-infective properties of prodiginines. Appl Microbiol Biotechnol 103(7):2873–2887. https://doi.org/10.1007/s00253-019-09641-1
Marchal E, Smithen DA, Uddin MI, Robertson AW, Jakeman DL, Mollard V, Goodman CD, MacDougall KS, McFarland SA, McFadden GI, Thompson A (2014) Synthesis and antimalarial activity of prodigiosenes. Org Biomol Chem 12(24):4132–4142. https://doi.org/10.1039/c3ob42548g
Ehrenkaufer G, Li P, Stebbins EE, Kangussu-Marcolino MM, Debnath A, White CV, Moser MS, DeRisi J, Gisselberg J, Yeh E, Wang SC, Company AH, Monti L, Caffrey CR, Huston CD, Wang B, Singh U (2020) Identification of anisomycin, prodigiosin and obatoclax as compounds with broad-spectrum anti-parasitic activity. PLoS Negl Trop Dis 14(3):e0008150. https://doi.org/10.1371/journal.pntd.0008150
Patil CD, Patil SV, Salunke BK, Salunkhe RB (2011) Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. Parasitol Res 109(4):1179–1187. https://doi.org/10.1007/s00436-011-2365-9
Rahul S, Chandrashekhar P, Hemant B, Chandrakant N, Laxmikant S, Satish P (2014) Nematicidal activity of microbial pigment from Serratia marcescens. Nat Prod Res 28(17):1399–1404. https://doi.org/10.1080/14786419.2014.904310
Suryawanshi RK, Patil CD, Borase HP, Narkhede CP, Salunke BK, Patil SV (2015) Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic Biochem Physiol 123:49–55. https://doi.org/10.1016/j.pestbp.2015.01.018
Offret C, Desriac F, Le Chevalier P, Mounier J, Jegou C, Fleury Y (2016) Spotlight on antimicrobial metabolites from the marine bacteria Pseudoalteromonas: chemodiversity and ecological significance. Mar Drugs 14(7). https://doi.org/10.3390/md14070129
Suzuki N, Ohtaguro N, Yoshida Y, Hirai M, Matsuo H, Yamada Y, Imamura N, Tsuchiya T (2015) A compound inhibits biofilm formation of Staphylococcus aureus from Streptomyces. Biol Pharm Bull 38(6):889–892. https://doi.org/10.1248/bpb.b15-00053
Akin-Osanaiye B, Aruwa I, Olobayotan I (2019) Isolation of Serratia marcescens from the soil and in vitro prodigiosin production as source of antibiotic, active against oxacillin-resistant Staphylococcus aureus. South Asian J Res Microbiol:1–9. https://doi.org/10.9734/SAJRM/2019/v4i430112
Zhang H, Wang H, Zheng W, Yao Z, Peng Y, Zhang S, Hu Z, Tao Z, Zheng T (2017) Toxic effects of prodigiosin secreted by Hahella sp. KA22 on harmful alga Phaeocystis globosa. Front Microbiol 8:999. https://doi.org/10.3389/fmicb.2017.00999
Gerber NN (1969) Prodigiosin-like pigments from Actinomadura (Nocardia) pelletieri and Actinomadura madurae. Appl Environ Microbiol 18(1):1–3. https://doi.org/10.1139/pmc377871
Ibrahim D, Nazari TF, Kassim J, Lim S-H (2014) Prodigiosin-an antibacterial red pigment produced by Serratia marcescens IBRL USM 84 associated with a marine sponge Xestospongia testudinaria. J Appl Pharm Sci 4:1–6. https://doi.org/10.7324/JAPS.2014.40101
Arivizhivendhan K, Mahesh M, Murali R, Mary RR, Thanikaivelan P, Sekaran G (2019) Prodigiosin–iron-oxide–carbon matrix for efficient antibiotic-resistant bacterial disinfection of contaminated water. ACS Sustain Chem Eng 7(3):3164–3175. https://doi.org/10.1021/acssuschemeng.8b05010
Mekhael R, Yousif S (2009) The role of red pigment produced by Serratia marcescens as antibacterial and plasmid curing agent. J Duhok Univ 12(1):268–274. https://doi.org/10.1007/s13205-017-0979-z
Berlanga M, Ruiz N, Hernandez-Borrell J, Montero T, Viñas M (2000) Role of the outer membrane in the accumulation of quinolones by Serratia marcescens. Can J Microbiol 46(8):716–722. https://doi.org/10.1007/pubmed/10941517
Woodhams DC, LaBumbard BC, Barnhart KL, Becker MH, Bletz MC, Escobar LA, Flechas SV, Forman ME, Iannetta AA, Joyce MD (2018) Prodigiosin, violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb Ecol 75(4):1049–1062. https://doi.org/10.1007/s00248-017-1095-7
Suryawanshi RK, Koujah L, Patil CD, Ames JM, Agelidis A, Yadavalli T, Patil SV, Shukla D (2020) Bacterial pigment prodigiosin demonstrates a unique anti-herpesvirus activity that is mediated through inhibition of prosurvival signal transducers. J Virol. https://doi.org/10.1128/JVI.00251-20
Danevčič T, Borić Vezjak M, Tabor M, Zorec M, Stopar D (2016) Prodigiosin induces autolysins in actively grown Bacillus subtilis cells. Front Microbiol 7:27. https://doi.org/10.3389/fmicb.2016.00027
Ravindran A, Anishetty S, Pennathur G (2020) Molecular dynamics of the membrane interaction and localisation of prodigiosin. J Mol Graph Model:107614. https://doi.org/10.1016/j.jmgm.2020.107614
Manderville R (2001) Synthesis, proton-affinity and anti-cancer properties of the prodigiosin-group natural products. Curr Med Chem Anticancer Agents 1(2):195–218. https://doi.org/10.2174/1568011013354688
Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP (2007) Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol 2(6):605–618. https://doi.org/10.2217/17460913.2.6.605
Lapenda J, Alves V, Adam M, Rodrigues M, Nascimento S (2020) Cytotoxic effect of prodigiosin, natural red pigment, isolated from Serratia marcescens UFPEDA 398. Indian J Microbiol:1–14. https://doi.org/10.1007/s12088-020-00859-6
Soto-Cerrato V, Llagostera E, Montaner B, Scheffer GL, Perez-Tomas R (2004) Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem Pharmacol 68(7):1345–1352. https://doi.org/10.1016/j.bcp.2004.05.056
Llagostera E, Soto-Cerrato V, Joshi R, Montaner B, Gimenez-Bonafé P, Pérez-Tomás R (2005) High cytotoxic sensitivity of the human small cell lung doxorubicin-resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anti-Cancer Drugs 16(4):393–399. https://doi.org/10.1097/00001813-200504000-00005
Baldino CM, Parr J, Wilson CJ, Ng S-C, Yohannes D, Wasserman HH (2006) Indoloprodigiosins from the C-10 bipyrrolic precursor: new antiproliferative prodigiosin analogs. Bioorg Med Chem Lett 16(3):701–704. https://doi.org/10.1016/j.bmcl.2005.10.027
Kataoka T, Muroi M, Ohkuma S, Waritani T, Magae J, Takatsuki A, Kondo S, Yamasaki M, Nagai K (1995) Prodigiosin 25-C uncouples vacuolar type H+-ATPase, inhibits vacuolar acidification and affects glycoprotein processing. FEBS Lett 359(1):53–59. https://doi.org/10.1016/0014-5793(94)01446-8
Francisco R, Pérez-Tomás R, Gimènez-Bonafé P, Soto-Cerrato V, Giménez-Xavier P, Ambrosio S (2007) Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur J Pharmacol 572(2-3):111–119. https://doi.org/10.1016/j.ejphar.2007.06.054
Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Madiraju SM, Goulet D, Viallet J, Bélec L, Billot X (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci 104(49):19512–19517. https://doi.org/10.1073/pnas.0709443104
Melvin MS, Tomlinson JT, Saluta GR, Kucera GL, Lindquist N, Manderville RA (2000) Double-strand DNA cleavage by copper prodigiosin. J Am Chem Soc 122(26):6333–6334. https://doi.org/10.1021/ja0000798
Melvin MS, Tomlinson JT, Park G, Day CS, Saluta GR, Kucera GL, Manderville RA (2002) Influence of the A-ring on the proton affinity and anticancer properties of the prodigiosins. Chem Res Toxicol 15(5):734–741. https://doi.org/10.1021/tx025507x
Hong B, Prabhu VV, Zhang S, van den Heuvel APJ, Dicker DT, Kopelovich L, El-Deiry WS (2014) Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res 74(4):1153–1165. https://doi.org/10.1158/0008-5472.CAN-13-0955
Zhao C, Qiu S, He J, Peng Y, Xu H, Feng Z, Huang H, Du Y, Zhou Y, Nie Y (2020) Prodigiosin impairs autophagosome-lysosome fusion that sensitizes colorectal cancer cells to 5-fluorouracil-induced cell death. Cancer Lett. https://doi.org/10.1016/j.canlet.2020.03.010
El-Batal AI, El-Hendawy HH, Faraag AH (2017) In silico and in vitro cytotoxic effect of prodigiosin-conjugated silver nanoparticles on liver cancer cells (HepG2). Cancer Lett 98(3). https://doi.org/10.5114/bta.2017.70801
Han SB, Park SH, Jeon YJ, Kim YK, Kim HM, Yang KH (2001) Prodigiosin blocks T cell activation by inhibiting interleukin-2Rα expression and delays progression of autoimmune diabetes and collagen-induced arthritis. J Pharmacol Exp Ther 299(2):415–425. https://doi.org/10.1002/pmc11602650
Han SB, Park SH, Jeon YJ, Kim YK, Kim HM, Yang KH (2001) Prodigiosin blocks T cell activation by inhibiting interleukin-2Ralpha expression and delays progression of autoimmune diabetes and collagen-induced arthritis. J Pharmacol Exp Ther 299(2):415–425. https://doi.org/10.1002/pmc11602650
Tomás P, Ricardo E, Montaner B (2003) Effects of the proapoptotic drug prodigiosin on cell cycle-related proteins in Jurkat T cells. Histol Histopathol 18(2):379–385. https://doi.org/10.14670/HH-18.379
Montaner B, Prez-Toms R (2003) The prodigiosins: a new family of anticancer drugs. Curr Cancer Drug Targets 3(1):57–65. https://doi.org/10.2174/1568009033333772
Songia S, Mortellaro A, Taverna S, Fornasiero C, Scheiber EA, Erba E, Colotta F, Mantovani A, Isetta A-M, Golay J (1997) Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J Immunol 158(8):3987–3995. https://doi.org/10.1002/pmc3987
Soto-Cerrato V, Viñals F, Lambert JR, Kelly JA, Pérez-Tomás R (2007) Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3β activity in human breast cancer cells. Mol Cancer Ther 6(1):362–369. https://doi.org/10.1158/1535-7163.MCT-06-0266
Sigurdson GT, Tang P, Giusti MM (2017) Natural colorants: food colorants from natural sources. Annu Rev Food Sci Technol 8:261–280. https://doi.org/10.1146/annurev-food-030216-025923
Marcus JB (2013) Vitamin and mineral basics: the ABCs of healthy foods and beverages, including phytonutrients and functional foods: healthy vitamin and mineral choices, roles and applications in nutrition, food science and the culinary arts. ch. 7. Food Science and the Culinary Arts. In: Marcus JB (ed) Culinary nutrition: the science and practice of healthy cooking. Academic Press, pp 279–331. https://doi.org/10.1016/B978-0-12-391882-6.00007-8
Edelmann M, Aalto S, Chamlagain B, Kariluoto S, Piironen V (2019) Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J Food Compos Anal 82:103226. https://doi.org/10.1016/j.jfca.2019.05.009
Bogacz-Radomska L, Harasym J (2018) β-Carotene—properties and production methods. Food Qual Saf 2(2):69–74. https://doi.org/10.1093/fqsafe/fyy004
Cong X-Y, Zhang H-Z (2019) Recent progress in sources, biological activity and application of astaxanthin. Int J Sci 8(03):31–34. https://doi.org/10.18483/ijSci.2011
Malis SA, Cohen E, Ben Amotz A (1993) Accumulation of canthaxanthin in Chlorella emersonii. Physiol Plant 87(2):232–236. https://doi.org/10.1111/j.1399-3054.1993.tb00148.x
Esatbeyoglu T, Rimbach G (2017) Canthaxanthin: from molecule to function. Mol Nutr Food Res 61(6). https://doi.org/10.1002/mnfr.201600469
Lee JS, Kim YS, Park S, Kim J, Kang SJ, Lee MH, Ryu S, Choi JM, Oh TK, Yoon JH (2011) Exceptional production of both prodigiosin and cycloprodigiosin as major metabolic constituents by a novel marine bacterium, Zooshikella rubidus S1-1. Appl Environ Microbiol 77(14):4967–4973. https://doi.org/10.1128/AEM.01986-10
Buchweitz M (2016) Natural solutions for blue colors in food. In: Handbook on natural pigments in food and beverages. Elsevier, pp 355–384. https://doi.org/10.1016/B978-0-08-100371-8.00017-8
Erandapurathukadumana Sreedharan H, Harilal CC, Pradeep S (2020) Response surface optimization of prodigiosin production by phthalate degrading Achromobacter denitrificans SP1 and exploring its antibacterial activity. Prep Biochem Biotechnol:1–8. https://doi.org/10.1080/10826068.2020.1712659
Gul S, Shad MA, Arshad R, Nawaz H, Ali A, Altaf A, Gul T, Iqbal W (2020) Response surface optimization of prodigiosin production by mutagen-treated Serratia marcescens in different growth media. Pharmacogn Mag 16(68):99. https://doi.org/10.4103/pm.pm_430_19
Kimata S, Matsuda T, Suizu Y, Hayakawa Y (2018) Prodigiosin R2, a new prodigiosin from the roseophilin producer Streptomyces griseoviridis 2464-S5. J Antibiot (Tokyo) 71(3):393–396. https://doi.org/10.1038/s41429-017-0011-1
Metwally RA, Nermeen A, El Sikaily A, Ghozlan HA, Sabry SA (2017) Statistical optimization and characterization of prodigiosin from a marine Serratia rubidaeaRAM_Alex. J Pure Appl Microbiol 11(3):1259–1266. https://doi.org/10.22207/JPAM.11.3.04
Kurbanoglu EB, Ozdal M, Ozdal OG, Algur OF (2015) Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. J Pure Appl Microbio 46(2):631–637. https://doi.org/10.1590/S1517-838246246220131143
Gondil VS, Asif M, Bhalla TC (2017) Optimization of physicochemical parameters influencing the production of prodigiosin from Serratia nematodiphila RL2 and exploring its antibacterial activity. 3. Biotech 7(5):338. https://doi.org/10.1007/s13205-017-0979-z
Xia S, Veony E, Yang Q (2018) Kitchen waste as a novel available substrate for prodigiosin production by Serratia marcescense. In: IOP Conference Series: Earth and Environmental Science, vol 1. IOP Publishing, p 012037. https://doi.org/10.1088/1755-1315/171/1/012037
Liang TW, Chen SY, Chen YC, Chen CH, Yen YH, Wang SL (2013) Enhancement of prodigiosin production by Serratia marcescens TKU011 and its insecticidal activity relative to food colorants. J Food Sci 78(11):M1743–M1751. https://doi.org/10.1111/1750-3841.12272
Wei YH, Chen WC (2005) Enhanced production of prodigiosin-like pigment from Serratia marcescens SMdeltaR by medium improvement and oil-supplementation strategies. J Biosci Bioeng 99(6):616–622. https://doi.org/10.1263/jbb.99.616
Liu X, Wang Y, Sun S, Zhu C, Xu W, Park Y, Zhou H (2013) Mutant breeding of Serratia marcescens strain for enhancing prodigiosin production and application to textiles. Prep Biochem Biotechnol 43(3):271–284. https://doi.org/10.1080/10826068.2012.721850
El-Bondkly AM, El-Gendy MM, Bassyouni RH (2012) Overproduction and biological activity of prodigiosin-like pigments from recombinant fusant of endophytic marine Streptomyces species. Antonie Van Leeuwenhoek 102(4):719–734. https://doi.org/10.1007/s10482-012-9772-5
Wei YH, Yu WJ, Chen WC (2005) Enhanced undecylprodigiosin production from Serratia marcescens SS-1 by medium formulation and amino-acid supplementation. J Biosci Bioeng 100(4):466–471. https://doi.org/10.1263/jbb.100.466
Montaner B, Pérez-Tomás R (2002) The cytotoxic prodigiosin induces phosphorylation of p38-MAPK but not of SAPK/JNK. Toxicol Lett 129(1-2):93–98. https://doi.org/10.1016/S0378-4274(01)00477-5
Soto-Cerrato V, Vinals F, Lambert JR, Perez-Tomas R (2007) The anticancer agent prodigiosin induces p21WAF1/CIP1 expression via transforming growth factor-beta receptor pathway. Biochem Pharmacol 74(9):1340–1349. https://doi.org/10.1016/j.bcp.2007.07.016
Wheler JJ, Janku F, Naing A, Li Y, Stephen B, Zinner R, Subbiah V, Fu S, Karp D, Falchook GS (2016) Cancer therapy directed by comprehensive genomic profiling: a single center study. Cancer Res 76(13):3690–3701. https://doi.org/10.1158/0008-5472
Castillo-Avila W, Abal M, Robine S, Perez-Tomas R (2005) Non-apoptotic concentrations of prodigiosin (H+/Cl- symporter) inhibit the acidification of lysosomes and induce cell cycle blockage in colon cancer cells. Life Sci 78(2):121–127. https://doi.org/10.1016/j.lfs.2005.04.059
Yenkejeh RA, Sam MR, Esmaeillou M (2017) Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum Exp Toxicol 36(4):402–411. https://doi.org/10.1177/0960327116651122
Chiu WJ, Lin SR, Chen YH, Tsai MJ, Leong MK, Weng CF (2018) Prodigiosin-emerged PI3K/Beclin-1-independent pathway elicits autophagic cell death in doxorubicin-sensitive and -resistant lung cancer. J Clin Med 7(10). https://doi.org/10.3390/jcm7100321
Liu Y, Zhou H, Ma X, Lin C, Lu L, Liu D, Ma D, Gao X, Qian XY (2018) Prodigiosin inhibits proliferation, migration, and invasion of nasopharyngeal cancer cells. Hum Exp Toxicol 48(4):1556–1562. https://doi.org/10.1159/000492278
Espona-Fiedler M, Soto-Cerrato V, Hosseini A, Lizcano JM, Guallar V, Quesada R, Gao T, Perez-Tomas R (2012) Identification of dual mTORC1 and mTORC2 inhibitors in melanoma cells: prodigiosin vs. obatoclax. Biochem Pharmacol 83(4):489–496. https://doi.org/10.1016/j.bcp.2011.11.027
Acknowledgment
The authors also acknowledge the cooperation and assistance received from the Department of Chemical engineering and Bio-engineering, National Institute of Technology, Agartala to conduct the reported study.
Funding
The authors received financial assistantship in the form of fellowship to conduct doctoral research from the National Institute of Technology and Ministry of Human Resource Development, Government of India.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Biosynthesis of prodigiosin and its source
• Extraction and purification of prodigiosin
• Biochemical characterization of prodigiosin
Rights and permissions
About this article
Cite this article
Paul, T., Bandyopadhyay, T.K., Mondal, A. et al. A comprehensive review on recent trends in production, purification, and applications of prodigiosin. Biomass Conv. Bioref. 12, 1409–1431 (2022). https://doi.org/10.1007/s13399-020-00928-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00928-2