Abstract
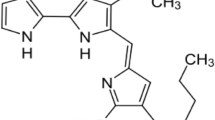
Since a decade, there has been a strong consumer demand for more natural products. This has augmented inclination towards substitution of synthetic colorants with natural pigments. Natural pigments not only have the capacity to increase the marketability of products, they also demonstrate valuable biological activities as antioxidants and anticancer agents. There is a long history of exploitation of natural products produced by bacteria as sources of pharmaceutically important, bioactive compounds. Among natural pigments, pigments from microbial sources are potentially suitable alternatives to synthetic pigments. The red pigment prodigiosin (PG) has unusual properties, which have long been documented. The red-pigmented prodiginines are bioactive secondary metabolites produced by both Gram-negative and Gram-positive bacteria. Prodigiosins are characterized by a common pyrrolyl pyrromethene skeleton, and the biological role of these pigments in the producer organisms remains unclear. Bacterial prodigiosins and their synthetic derivatives are effective proapoptotic agents against various cancer cell lines, with multiple cellular targets including multi-drug resistant cells with little or no toxicity towards normal cell lines. However, research into the biology of pigment production will stimulate interest in the bioengineering of strains to synthesize useful prodiginine derivatives. This review article highlights the characteristics and potential applications of prodigiosin pigment from Serratia as prodigiosins are real potential therapeutic drugs.




Similar content being viewed by others
References
Ahmad WA, Wan Ahmad WY, Zakaria ZA, Yusof NZ (2012) Appl Bact Pigments Colorant. doi:10.1007/978-3-642-24520-6
Baldino CM, Parr J, Wilson CJ et al (2006) Indoloprodigiosins from the C-10 bipyrrolic precursor: new antiproliferative prodigiosin analogs. Bioorg Med Chem Lett 16:701–4. doi:10.1016/j.bmcl.2005.10.027
Berlanga M, Ruiz N, Hernandez-Borrell J et al (2000) Role of the outer membrane in the accumulation of quinolones by Serratia marcescens. Can J Microbiol 46:716–22
Browning DF, Whitworth DE, Hodgson DA (2003) Light-induced carotenogenesis in Myxococcus xanthus: functional characterization of the ECF sigma factor CarQ and antisigma factor CarR. Mol Microbiol 48:237–51
Burke C, Thomas T, Egan S, Kjelleberg S (2007) The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ Microbiol 9:814–8. doi:10.1111/j.1462-2920.2006.01177.x
Campàs C, Dalmau M, Montaner B et al (2003) Prodigiosin induces apoptosis of B and T cells from B-cell chronic lymphocytic leukemia. Leukemia 17:746–50. doi:10.1038/sj.leu.2402860
Cerdeño AM, Bibb MJ, Challis GL (2001) Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): new mechanisms for chain initiation and termination in modular multienzymes. Chem Biol 8:817–829
Coco EA, Narva KE, Feitelson JS (1991) New classes of Streptomyces coelicolor A3(2) mutants blocked in undecylprodigiosin (Red) biosynthesis. Mol Gen Genet 227:28–32
Cross BE, Edinberry MN, Turner WB (1972) Pigments of Gnomonia erythrostoma. Part I. The structures of erythrostominone, deoxyerythrostominone, and deoxyerythrostominol. J Chem Soc Perkin Trans 1:380. doi:10.1039/p19720000380
Cserháti T (2006) Liquid chromatography of natural pigments and synthetic dyes. 602
Darah Ibrahim TFNJKS-HL (2014) Prodigiosin - an antibacterial red pigment produced by Serratia marcescens IBRL USM 84 associated with a marine sponge Xestospongia testudinaria. J Appl Pharm Sci 4:001–006
Francisco R, Pérez-Tomás R, Gimènez-Bonafé P et al (2007) Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur J Pharmacol 572:111–9. doi:10.1016/j.ejphar.2007.06.054
Fürstner A, Grabowski J, Lehmann CW et al (2001) Synthesis and biological evaluation of nonylprodigiosin and macrocyclic prodigiosin analogues. Chembiochem 2:60–8
Garneau-Tsodikova S, Dorrestein PC, Kelleher NL, Walsh CT (2006) Protein assembly line components in prodigiosin biosynthesis: characterization of PigA, G, H, I, J. J Am Chem Soc 128:12600–1. doi:10.1021/ja063611l
Gerber NN (1969) Prodigiosin-like pigments from Actinomadura (Nocardia) pelletieri and Actinomadura madurae. Appl Microbiol 18:1–3
Giri AV, Anandkumar N, Muthukumaran G, Pennathur G (2004) A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol 4:11. doi:10.1186/1471-2180-4-11
Golubev WI (1995) Perfect state of Rhodomyces dendrorhous (Phaffia rhodozyma). Yeast 11:101–10. doi:10.1002/yea.320110202
Guryanov ID, Karamova NS, Yusupova D V., et al (2013) Bacterial pigment prodigiosin and its genotoxic effect. Russ J Bioorganic Chem 39:106–111. doi:10.1134/S1068162012060040
Guthrie EP, Flaxman CS, White J et al (1998) A response-regulator-like activator of antibiotic synthesis from Streptomyces coelicolor A3(2) with an amino-terminal domain that lacks a phosphorylation pocket. Microbiology 144(Pt 3):727–38
Han SB, Park SH, Jeon YJ et al (2001) Prodigiosin blocks T cell activation by inhibiting interleukin-2Ralpha expression and delays progression of autoimmune diabetes and collagen-induced arthritis. J Pharmacol Exp Ther 299:415–25
Harris AKP, Williamson NR, Slater H et al (2004) The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology 150:3547–60. doi:10.1099/mic. 0.27222-0
Hong B, Prabhu VV, Zhang S et al (2014) Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res 74:1153–65. doi:10.1158/0008-5472.CAN-13-0955
Hubbard R, Rimington C (1950) The biosynthesis of prodigiosin, the tripyrrylmethene pigment from Bacillus prodigiosus (Serratia marcescens). Biochem J 46:220–5
Joshi VK, Attri D, Baja A, Bhushan S (2003) Microb Pigments 2:362–369
Kalesperis GS, Prahlad KV, Lynch DL (1975) Toxigenic studies with the antibiotic pigments from Serratia marcescens. Can J Microbiol 21:213–20
Kataoka T, Muroi M, Ohkuma S et al (1995) Prodigiosin 25-C uncouples vacuolar type H(+)-ATPase, inhibits vacuolar acidification and affects glycoprotein processing. FEBS Lett 359:53–59
Kawasaki T, Sakurai F, Nagatsuka S, Hayakawa Y (2009) Prodigiosin biosynthesis gene cluster in the roseophilin producer Streptomyces griseoviridis. J Antibiot (Tokyo) 62:271–6. doi:10.1038/ja.2009.27
Khanafari A, Assadi MM, Fakhr FA (2006) Review of prodigiosin, pigmentation in Serratia marcescens Qods Sqr ., Tajrish Sqr . Tehran, Iran Department of Forest Sciences, Faculty of Forestry, The University of British Columbia, 4th Floor Forest Sciences Centre # 4320–2424 Main Mall Vancouver. 6:1–13
Kim D, Lee JS, Park YK et al (2007) Biosynthesis of antibiotic prodiginines in the marine bacterium Hahella chejuensis KCTC 2396. J Appl Microbiol 102:937–944. doi:10.1111/j.1365-2672.2006.03172.x
Llagostera E, Soto-Cerrato V, Joshi R et al (2005) High cytotoxic sensitivity of the human small cell lung doxorubicin-resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anticancer Drugs 16:393–9
Malik K, Tokkas J, Goyal S (2012) Microbial Pigments: a review. 361–365
Malpartida F, Niemi J, Navarrete R, Hopwood DA (1990) Cloning and expression in a heterologous host of the complete set of genes for biosynthesis of the Streptomyces coelicolor antibiotic undecylprodigiosin. Gene 93:91–9
Manderville RA (2001) Synthesis, proton-affinity and anti-cancer properties of the prodigiosin-group natural products. Curr Med Chem Anticancer Agents 1:195–218
Mekhael R, Samira Y (2009) The role of red pigment produced by Serratia marcescens AS. J Duhok Univ 12(No1 (Special Issue) 12):268–274
Melvin MS, Tomlinson JT, Saluta GR et al (2000) Double-strand DNA cleavage by Copper•Prodigiosin. J Am Chem Soc 122:6333–6334. doi:10.1021/ja0000798
Melvin MS, Tomlinson JT, Park G et al (2002) Influence of the A -ring on the proton affinity and anticancer properties of the prodigiosins. Chem Res Toxicol 15:734–741. doi:10.1021/tx025507x
Mizukami H, Konoshima M, Tabata M (1978) Variation in pigment production in Lithospermum erythrorhizon callus cultures. Phytochemistry 17:95–97. doi:10.1016/S0031-9422(00)89687-9
Mo S, Sydor PK, Corre C et al (2008) Elucidation of the Streptomyces coelicolor pathway to 2-undecylpyrrole, a key intermediate in undecylprodiginine and streptorubin B biosynthesis. Chem Biol 15:137–48. doi:10.1016/j.chembiol.2007.11.015
Montaner B, Pérez-Tomás R (2003) The prodigiosins: a new family of anticancer drugs. Curr Cancer Drug Targets 3:57–65
Nagpal N, Munjal N, Chatterjee S (2011) Microbial pigments with health benefits—a mini review. Trends Biosci 4:157–160
Namazkar S, Ahmad WA (2013) Spray-dried prodigiosin from Serratia marcescens as a colorant. Biosci Biotechnol Res Asia 10:69–76. doi:10.13005/bbra/1094
Narva KE, Feitelson JS (1990) Nucleotide sequence and transcriptional analysis of the redD locus of Streptomyces coelicolor A3(2). J Bacteriol 172:326–33
Nguyen M, Marcellus RC, Roulston A et al (2007) Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A 104:19512–7. doi:10.1073/pnas.0709443104
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54:287–301
Pérez-Tomás R, Montaner B (2003) Effects of the proapoptotic drug prodigiosin on cell cycle-related proteins in Jurkat T cells. Histol Histopathol 18:379–85
Pryce LH, Terry FW (2000) Spectrophotometric assay of gene expression: Serratia marcescens pigmentation. Bioscene 26:3–13
Rapoport H, Holden KG (1962) The synthesis of prodigiosin. J Am Chem Soc 84:635–642. doi:10.1021/ja00863a026
Ryazantseva I, Andreyeva I (2014) Application of prodigiosin as a colorant for polyolefines. Adv Biol Chem 04:20–25. doi:10.4236/abc.2014.41004
Schloss PD, Allen HK, Klimowicz AK et al (2010) Psychrotrophic strain of Janthinobacterium lividum from a cold Alaskan soil produces prodigiosin. DNA Cell Biol 29:533–41. doi:10.1089/dna.2010.1020
Singh P, Shekhawat N (2012) chemometric descriptors in the rationale of antimalarial activity of natural and synthetic prodiginines. 2:244–260
Songia S, Mortellaro A, Taverna S et al (1997) Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J Immunol 158:3987–95
Soto-Cerrato V, Llagostera E, Montaner B et al (2004) Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem Pharmacol 68:1345–1352. doi:10.1016/j.bcp.2004.05.056
Soto-Cerrato V, Viñals F, Lambert JR et al (2007) Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3beta activity in human breast cancer cells. Mol Cancer Ther 6:362–9. doi:10.1158/1535-7163.MCT-06-0266
Stahmann KP, Revuelta JL, Seulberger H (2000) Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl Microbiol Biotechnol 53:509–16
Stanley AE, Walton LJ, Kourdi Zerikly M, et al. (2006) Elucidation of the Streptomyces coelicolor pathway to 4-methoxy-2,2’-bipyrrole-5-carboxaldehyde, an intermediate in prodiginine biosynthesis. Chem Commun (Camb) 3981–3. doi: 10.1039/b609556a
Suryawanshi RK, Patil CD, Borase HP et al (2014) Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl Biochem Biotechnol 173:1209–21. doi:10.1007/s12010-014-0921-3
Takano H, Obitsu S, Beppu T, Ueda K (2005) Light-induced carotenogenesis in Streptomyces coelicolor A3(2): identification of an extracytoplasmic function sigma factor that directs photodependent transcription of the carotenoid biosynthesis gene cluster. J Bacteriol 187:1825–32. doi:10.1128/JB.187.5.1825-1832.2005
Thomas MG, Burkart MD, Walsh CT (2002) Conversion of L-proline to pyrrolyl-2-carboxyl-S-PCP during undecylprodigiosin and pyoluteorin biosynthesis. Chem Biol 9:171–184
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–10. doi:10.1038/35042675
Walsh CT, Garneau-Tsodikova S, Howard-Jones AR (2006) Biological formation of pyrroles: nature’s logic and enzymatic machinery. Nat Prod Rep 23:517–31. doi:10.1039/b605245m
Wasserman HH, McKeon JE, Smith L, Forgione P (1960) prodigiosin. Structure and partial synthesis 1. J Am Chem Soc 82:506–507. doi:10.1021/ja01487a075
White J, Bibb M (1997) bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J Bacteriol 179:627–33
Williams RP (1973) Biosynthesis of prodigiosin, a secondary metabolite of Serratia marcescens. Appl Microbiol 25:396–402
Williams RP, Green JA, Rappo-Port DA (1956) Studies on pigmentation of Serratia marcescens. I. Spectral and paper chromatographic properties of prodigiosin. J Bacteriol 71:115–20
Williamson NR, Simonsen HT, Ahmed RAA et al (2005) Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol Microbiol 56:971–89. doi:10.1111/j.1365-2958.2005.04602.x
Williamson NR, Fineran PC, Leeper FJ, Salmond GPC (2006) The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol 4:887–899. doi:10.1038/nrmicro1531
Williamson NR, Fineran PC, Gristwood T et al (2007) Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol 2:605–618
Yamamoto C, Takemoto H, Kuno K et al (1999) Cycloprodigiosin hydrochloride, a new H(+)/Cl(−) symporter, induces apoptosis in human and rat hepatocellular cancer cell lines in vitro and inhibits the growth of hepatocellular carcinoma xenografts in nude mice. Hepatology 30:894–902. doi:10.1002/hep.510300417
Yamamoto D, Uemura Y, Tanaka K et al (2000) Cycloprodigiosin hydrochloride, H(+)/CL(−) symporter, induces apoptosis and differentiation in HL-60 cells. Int J Cancer 88:121–8
Yokoyama A, Izumida H, Miki W (1994) Production of astaxanthin and 4-Ketozeaxanthin by the marine bacterium, Agrobacterium aurantiacum. Biosci Biotechnol Biochem 58:1842–1844. doi:10.1271/bbb.58.1842
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darshan, N., Manonmani, H.K. Prodigiosin and its potential applications. J Food Sci Technol 52, 5393–5407 (2015). https://doi.org/10.1007/s13197-015-1740-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1740-4