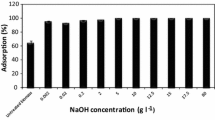
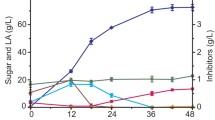
Abstract
Immobilization is a simple technique in which microorganisms adhere and agglomerate onto the porous material’s surface, resulting in a higher cell density, cell tolerance, and productivity. Acetone-butanol-ethanol (ABE) fermentation using immobilized Clostridium beijerinckii JCM 8026 on Napier grass (Pennisetum purpureum) chemically pretreated with H2SO4, NaOH, and 1-ethyl-3-methylimidazolium acetate (EMIM-OAc) was compared in order to investigate the effect of the treatment on butanol production. X-ray diffraction, Fourier-transform infrared spectroscopy, and surface area measurement analyses indicated that the alkaline-pretreated Napier grass sample had the highest crystalline level with the lowest lignin content (lignin/cellulose) and a high surface area compared with other pretreated samples, suggesting its potential as an immobilized material. The fermentation was operated in a batch system for 5 days with an initial glucose level of 60 g/L. Clostridium beijerinckii JCM 8026 immobilized on the NaOH-treated Napier grass gave the highest butanol concentration (8.99 g/L), which corresponded to a 24.7% and 25.6% higher concentration than that when the cells were immobilized on untreated Napier grass and free cell culture, respectively. It is likely that immobilization on NaOH-treated Napier grass increased the cells’ protection from environmental stresses and prevented their washing out due to its swollen structure within an enlarged surface area.







Similar content being viewed by others
References
Lee S, Park J, Jang S, Nielsen L, Kim J, Jung K (2008) Fermentative butanol production by clostridia. Biotechnol Bioeng 101(2):209–228. https://doi.org/10.1002/bit.22003
Wang Y, Guo W, Cheng C-L, Ho S-H, Chang J-S, Ren N (2016) Enhancing bio-butanol production from biomass of Chlorella vulgaris JSC-6 with sequential alkali pretreatment and acid hydrolysis. Bioresour Technol 200 (Supplement C: 557–564. https://doi.org/10.1016/j.biortech.2015.10.056
Dϋrre P (2008) Fermentative butanol production bulk chemical and biofuel. Ann N Y Acad Sci 1125(1):353–362. https://doi.org/10.1196/annals.1419.009
Qureshi N, Saha BC, Hector RE, Hughes SR, Cotta MA (2008) Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: part I-batch fermentation. Biomass Bioenergy 32(2):168–175. https://doi.org/10.1016/j.biombioe.2007.07.004
Ezeji TC, Qureshi N, Blaschek HP (2007) Bioproduction of butanol from biomass: from genes to bioreactors. Biotechnol 18(3):220–227. https://doi.org/https://doi.org/10.1016/j.copbio.2007.04.002
Ezeji TC, Milne C, Price ND, Blaschek HP (2010) Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol producing microorganisms. Appl Microbiol Biotechnol 85(6):1697–1712. https://doi.org/10.1007/s00253-009-2390-0
Yen HW, Li RJ (2011) The effects of dilution rate and glucose concentration on continuous acetone–butanol–ethanol fermentation by Clostridium acetobutylicum immobilized on bricks. J Chem Technol Biotechnol 86(11):1399–1404. https://doi.org/10.1002/jctb.2640
Zhang Y, Ma Y, Yang F, Zhang C (2009) Continuous acetone–butanol–ethanol production by corn stalk immobilized carrier. J Ind Microbiol Biotechnol 36(8):1117–1121. https://doi.org/10.1007/s10295-009-0582-3
Costa F, Silva B, Tavares T (2017) Chapter 6 – Biofilm bioprocesses. In: Sanromán MÁ, Du G, Pandey A (eds) Current developments in biotechnology and bioengineering, Elsevier, pp 143—175. https://doi.org/https://doi.org/10.1016/B978-0-444-636638.00006-9
Djukic-Vukovic AP, Mojovic LV, Jokic BM, Nikolic SB, Pejin JD (2013) Lactic acid production on liquid distillery stillage by Lactobacillus rhamnosus immobilized onto zeolite. Bioresour Technol 135(Supplement C):454–458. https://doi.org/https://doi.org/10.1016/j.biortech.2012.10.066, 135
Vichuviwat R, Boonsombuti A, Luengnaruemitchai A, Wongkasemjit S (2014) Enhanced butanol production by immobilized Clostridium beijerinckii TISTR 1461 using zeolite 13X as a carrier. Bioresour Technol 172:76–82. https://doi.org/10.1016/j.biortech.2014.09.008
Passos F, Uggetti E, Carrere H and Ferrer I (2014) Pretreatment of microalgae to improve biogas production: a review. Bioresour Technol 172:403–412. https://doi.org/https://doi.org/10.1016/j.biortech.2014.08.114
Zhou W, Liu J, Fan S, Xiao Z, Qiu B, Wang Y, Li J, Liu Y (2018) Biofilm immobilization of Clostridium acetobutylicum on particulate carriers for acetone-butanol-ethanol (ABE) production. Bioresour Technol Rep 3:211–217. https://doi.org/10.1016/j.biteb.2018.08.008
Mohammed IY, Abakr YA, Kazi FK, Yusup S, Alshareef I, Chin SA (2015) Comprehensive characterization of Napier grass as a feedstock for thermochemical conversion. Energies 8(5):3403–3417. https://doi.org/. https://doi.org/10.3390/en8053403
Boonsombuti A, Wanapirom R, Luengnaruemitchai A, Wongkasemjit S (2018) The effect of the addition of acetic acid to aqueous ionic liquid mixture using microwave-assisted pretreatment in the saccharification of Napier grass. Waste Biomass Valori 9(10):1795–1804. https://doi.org/10.1007/s12649-017-9908-y
Eliana C, Jorge R, Juan P, Luis R (2014) Effects of the pretreatment method on enzymatic hydrolysis and ethanol fermentability of the cellulosic fraction from elephant grass. Fuel 118:41–47. https://doi.org/10.1016/j.fuel.2013.10.055
Tsai M-H, Lee W-C, Kuan W-C, Sirisansaneeyakul S, Savarajara A (2018) Evaluation of different pretreatments of Napier grass for enzymatic saccharification and ethanol production. Energy Sci Eng 6(6):683–692. https://doi.org/10.1002/ese3.243
Cardoso WS, Tardin FD, Tavares GP, Queiroz PV, Mota SS, Kasuya MCM, JHd Q (2013) Use of sorghum straw (Sorghum bicolor) for second generation ethanol production: pretreatment and enzymatic hydrolysis. Química Nova 36:623–627. https://doi.org/10.1590/S0100-40422013000500002
Arora R, Manisseri C, Li C, Ong MD, Scheller HV, Vogel K, Simmons BA, Singh S (2010) Monitoring and analyzing process streams towards understanding ionic liquid pretreatment of switchgrass (Panicum virgatum L.). BioEnergy Res 3(2):134–145. https://doi.org/10.1007/s12155-010-9087-1
Qureshi N, Blaschek HP (1999) Butanol recovery from model solution/fermentation broth by pervaporation: evaluation of membrane performance. Biomass Bioenergy 17(2):175–184. https://doi.org/10.1016/S0961-9534(99)00030-6
Segal L, Creely JJ, AE Martin Jr, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Manaso J, Luengnaruemitchai A, Wongkasemjit S (2013) Optimization of two-stage pretreatment combined with microwave radiation using response surface methodology. Int J Chem Mol Nucl Mater Metall Eng 7(4):191–196. https://doi.org/10.5281/zenodo.1081896
Chen WH, Tu YJ, Sheen HK (2011) Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave assisted heating. Appl Energy 88(8):2726–2734. https://doi.org/10.1016/j.apenergy.2011.02.027
Zhang Q, Hu J, Lee D-J (2017) Pretreatment of biomass using ionic liquids: research updates. Renew Energy 111:77–84. https://doi.org/10.1016/j.renene.2017.03.093
Luengnaruemitchai A, Anupapwisetkul C (2019) Surface morphology and cellulose structure of Napier grass pretreated with the ionic liquid 1-ethyl-3-methylimidazolium acetate combined with either water or dimethyl sulfoxide as a co-solvent under microwave irradiation. Biomass Convers Bior In press. https://doi.org/10.1007/s13399-019-00422-4
Singh R, Tiwari S, Srivastava M, Shukla A (2014) Microwave assisted alkali pretreatment of rice straw for enhancing enzymatic digestibility. J Energy 2014:1–7. https://doi.org/10.1155/2014/483813
Yang H, Yan R, Chen H, Lee DH, Zheng C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86(12):1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Chen Y, Zhou T, Liu D, Li A, Xu SB, Liu QG, Li BB, Ying HJ (2013) Production of butanol from glucose and xylose with immobilized cells of Clostridium acetobutylicum. Biotechnol Bioprocess Eng 18(2):234–241. https://doi.org/10.1007/s12257-012-0573-5
Poletto M, Pistor V, Zattera AJ (2013) Structural characteristics and thermal properties of native cellulose. In: Ven TVD, Godbout L (eds) Cellulose – Fundamental Aspects, IntechOpen pp 45–68. https://doi.org/https://doi.org/10.5772/50452
Wada M, Ike M, Tokuyasu K (2010) Enzymatic hydrolysis of cellulose I is greatly accelerated via its conversion to the cellulose II hydrate form. Polym Degrad Stab 95(4):543–548. https://doi.org/10.1016/j.polymdegradstab. 2009.12.014
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199(Supplement C):49–58. https://doi.org/10.1016/j.biortech
Jin Z, Katsumata KS, Lam TBT, Iiyama K (2006) Covalent linkages between cellulose and lignin in cell walls of coniferous and nonconiferous woods. Biopolymers 83(2):103–110. https://doi.org/10.1002/bip.20533
Zhou Y, Stuart-Williams H, Farquhar GD, Hocart CH (2010) The use of natural abundance stable isotopic ratios to indicate the presence of oxygen-containing chemical linkages between cellulose and lignin in plant cell walls. Phytochemistry 71(8):982–993. https://doi.org/10.1016/j.phytochem.2010.03.001
Spiridon I, Teaca CA, Bodîrlău R (2011) Structural changes evidenced by FTIR spectroscopy in cellulose materials after pre-treatment with ionic liquid and enzymatic hydrolysis. Bioresources 6(1):400–413. https://doi.org/10.15376/biores.6.1.400-413
Qiu Z, Aita GM, Walker MS (2012) Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresour Technol 117(Supplement C) 117:251–256. https://doi.org/10.1016/j.biortech.2012.04.070
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M, Vogel KP, Simmons BA, Singh S (2010) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. Bioresour Technol 101(13):4900–4906. https://doi.org/10.1016/j.biortech.2009.10.066
Cai L-Y, Ma Y-L, Ma X-X, Lv J-M (2016) Improvement of enzymatic hydrolysis and ethanol production from corn stalk by alkali and N-methylmorpholine-N-oxide pretreatments. Bioresour Technol 212:42–46. https://doi.org/10.1016/j.biortech.2016.04.012
Hosseinaei O, Wang S, Rials TG, Xing C, Zhang Y (2011) Effects of decreasing carbohydrate content on properties of wood strands. Cellulose 18(3):841–850. https://doi.org/10.1007/s10570-011-9519-x
Reddy N, Yang Y (2009) Properties and potential applications of natural cellulose fibers from the bark of cotton stalks. Bioresour Technol 100(14):3563–3569. https://doi.org/10.1016/j.biortech.2009.02.047
Sim SF, Mohamed M, Mohd Irwan Lu NAL, P Sarman NS, Samsudin SNS (2012) Computer-assisted analysis of Fourier transform infrared (FTIR) spectra for characterization of various treated and untreated agriculture biomass. Bioresources 7(4):5367–5380. https://doi.org/10.15376/biores.7.4.5367-5380
Xu F, Yu J, Tesso T, Dowell F, Wang D (2013) Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: a mini-review. Appl Energy 104(Supplement C) 104:801–809. https://doi.org/10.1016/j.apenergy.2012.12.019
Zeng Y, Zhao S, Yang S, Ding S-Y (2014) Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol 27:38–45. https://doi.org/10.1016/j.copbio.2013.09.008
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50(4):484–524
Ls K, Jn S, Mr S, Zhibin L, Baiquan C, Sharma V, Sharma PK (2013) Biobutanol from renewable agricultural and lignocellulose resources and its perspectives as alternative of liquid fuels. In: Fang Z (ed) Liquid, gaseous and solid biofuels - conversion techniques, IntechOpen, pp 199–262. https://doi.org/10.5772/52379
Jin C, Yao M, Liu H, Lee C-fF, Ji J (2011) Progress in the production and application of n-butanol as a biofuel. Renew Sust Energ Rev 15(8):4080–4106. https://doi.org/10.1016/j.rser.2011.06.001
Zhu Y (20017) Chapter 14 - Immobilized cell fermentation for production of chemicals and fuels. In: Yang ST (ed) Bioprocessing for value-added products from renewable resources. Elsevier, Amsterdam, pp 373–396. https://doi.org/10.1016/B978-044452114-9/50015-3
Huang W-C, Ramey DE, Yang S-T (2004) Continuous production of butanol by Clostridium acetobutylicum immobilized in a fibrous bed bioreactor. Appl Biochem Biotechnol 115(1):887–898. https://doi.org/10.1385/ABAB:115:1-3:0887
Cai D, Li P, Chen C, Wang Y, Hu S, Cui C, Qin P, Tan T (2016) Effect of chemical pretreatments on corn stalk bagasse as immobilizing carrier of Clostridium acetobutylicum in the performance of a fermentation-pervaporation coupled system. Bioresour Technol 220:68–75. https://doi.org/10.1016/j.biortech.2016.08.049
Chang Z, Cai D, Wang C, Li L, Han J, Qin P, Wang Z (2014) Sweet sorghum bagasse as an immobilized carrier for ABE fermentation by using Clostridium acetobutylicum ABE 1201. RSC Adv 4(42):21819–21825. https://doi.org/10.1039/C4RA00187G
Loyarkat S, Cheirsilp B, Praasertsan P (2015) Two-stage repeated-batch fermentation of immobilized Clostridium beijerinckii on oil palm fronds for solvents production. Process Biochem 50(8):1167–1176. https://doi.org/10.1016/j.procbio.2015.04.016
Funding
This research was funded by Chulalongkorn University (CU-GES-60-04-63-03) and by the Government budget (GB-A_61_046_63_05), Thailand. The authors acknowledge the 100th Anniversary Chulalongkorn University for Doctoral Scholarship, the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), and the Energy Conservation Fund (ENCON Fund) from Energy Policy and Planning Office (EPPO), Thailand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chinwatpaiboon, P., Doolayagovit, I., Boonsombuti, A. et al. Comparison of acid-, alkaline-, and ionic liquid–treated Napier grass as an immobilization carrier for butanol production by Clostridium beijerinckii JCM 8026. Biomass Conv. Bioref. 10, 1071–1082 (2020). https://doi.org/10.1007/s13399-019-00491-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00491-5