Abstract
Fundamental understanding of biomass pretreatment and its influence on saccharification kinetics, total sugar yield, and inhibitor formation is essential to develop efficient next-generation biofuel strategies, capable of displacing fossil fuels at a commercial level. In this study, we investigated the effect of residence time and temperature during ionic liquid (IL) pretreatment of switchgrass using 1-ethyl-3-methyl imidazolium acetate. The primary metrics of pretreatment performance are biomass delignification, xylan and glucan depolymerization, porosity, surface area, cellulase kinetics, and sugar yields. Compositional analysis and quantification of process streams of saccharides and lignin demonstrate that delignification increases as a function of pretreatment temperature and is hypothesized to be correlated with the apparent glass transition temperature of lignin. IL pretreatment did not generate monosaccharides from hemicellulose. Compared to untreated switchgrass, Brunauer–Emmett–Teller surface area of pretreated switchgrass increased by a factor of ∼30, with a corresponding increase in saccharification kinetics of a factor of ∼40. There is an observed dependence of cellulase kinetics with delignification efficiency. Although complete biomass dissolution is observed after 3 h of IL pretreatment, the pattern of sugar release, saccharification kinetics, and total sugar yields are strongly correlated with temperature.
Similar content being viewed by others
Introduction
With the prospect of diminishing fossil fuel supplies and estimated high levels of atmospheric carbon dioxide projected to reach 600 ppm by year 2035 (http://www.occ.gov.uk/activities/stern.htm, Office of Climate Change, UK), there is a great urgency for developing carbon neutral and renewable sources of transportation fuels. Advanced biofuels derived from lignocellulosic biomass are a potential source of renewable transportation fuel, with significantly reduced carbon emissions. Lignocellulosic biomass is composed mainly of cellulose, hemicellulose, and lignin. Cellulose is the most abundant polymer on earth and is composed of fermentable glucose that can be readily converted into biofuel [1]. However, the glucose is hard to liberate cost-effectively due to extensive intermolecular and intramolecular hydrogen bonding of the β-1,4-glycan chains in crystalline cellulose [2]. Hemicellulose is rich in xylose and interacts with cellulose and lignin to strengthen the cell wall. The interactions are mostly noncovalent, but in grasses the arabinoxylan can be covalently linked to lignin through oxidative coupling via ferulate esters [3]. The lignin is a polymer of monolignols, which are phenolic alcohols derived from coumaric acid. Lignin plays an essential role in plants by giving strength to stems and by making tracheary elements watertight and able to withstand the negative pressure in the xylem. Lignin is very difficult to break down and makes polysaccharides inaccessible to enzymes.
Before enzymatic saccharification, lignocellulosic biomass must be pretreated to increase the accessibility of the polysaccharides to hydrolytic enzymes [4]. After pretreatment, enzyme cocktails are capable of hydrolyzing the polysaccharides into simple sugars (C6 and C5) [5] but are currently very expensive. It is therefore crucial that pretreatment methods enable the saccharification to take place without excessive amounts of enzyme. The bond energies of the massive hydrogen bonding network present in cellulose can reach more than 23–25 kJ/mol and render traditional solvents unsuitable for effective biomass dissolution [6, 7]. Various pretreatment technologies are currently being tested and show some promise but face significant commercialization challenges due to an incremental enhancement in saccharification kinetics, requisite high temperature and/or pressure, and overall process economics [8–10].
Recently, IL pretreatment has been shown to be a promising pretreatment technology due to its ability to solubilize biomass by overcoming the hydrogen bonding within cellulose [10–14]. Other benefits of IL pretreatment include efficient precipitation and recovery of dissolved polysaccharides upon addition of antisolvent and desirable solvent attributes like low volatility, nonflammability, and thermal stability [10–14]. In order for ILs to develop beyond these initial positive results into an effective and commercial biomass pretreatment, three main conditions must be met: (1) solubilization of bioenergy crops at high biomass loading (20–30 wt.%), (2) solvent recovery and recycling, and (3) minimal generation of inhibitory by-products that may render cellulolytic enzymes and fermentation microbes inactive.
Towards those goals, a significant amount of research and technology development are needed to understand the nature and extent of biomass solubilization. It has been shown that crystalline cellulose is converted to an amorphous structure during IL pretreatment [10, 15, 16], but the extent of cellulose depolymerization is not sufficiently known. Similarly, the impact of IL pretreatment on hemicellulose is unknown. High-temperature acid pretreatments are very effective in converting hemicellulose to simple sugars but lead to ring opening of glucose and xylose [17, 18] that produce the known microbial inhibitors furfural and hydroxyl methyl furfural [19, 20]. The association of lignin with the polysaccharides has been linked directly to biomass recalcitrance [10, 21, 22]. Although ILs have been shown to be very effective in cellulose solubilization, the fate (referred as disposition in this article) of the lignin carbohydrate complex is not understood. The aim of this study was to develop a fundamental understanding of IL pretreatment by monitoring and analyzing process streams (Fig. 1). The development and optimization of IL pretreatment conditions for the selective depolymerization of either cellulose or lignin, whereby fractionation of different cellulosic and lignin components could be realized, are of great importance and the subject of this study.
Detailed parametric studies of temperature and residence time were carried out for a promising IL for biomass solubilization, 1-ethyl-3-methyl imidazolium acetate (abbreviated as [C2mim][OAc]) [23]. We have selected a potential dedicated energy crop switchgrass (Panicum virgatum L.). Switchgrass is native to North America; it is drought resistant and can grow over 1.8 m tall. It is presently used for forage production and soil conservation and has shown potential for biofuel production [24–28]. The recovered biomass upon antisolvent addition was analyzed in terms of delignification, porosity, surface area, total sugar yield after saccharification, and initial saccharification kinetics. The liquid from the process stream was analyzed for total monomeric sugar yields of cellulose, hemicellulose, xylose, arabinose, glucuronic acid, and other minor C5 and C6 sugars.
Materials and Methods
Plant Material
Switchgrass was obtained from Ken Vogel at the US Department of Agriculture, Lincoln, NE, USA. It was milled with a Thomas-Wiley Mini Mill fitted with a 40-mesh screen (Model 3383-L10 Arthur H. Thomas Co., Philadelphia, PA, USA).
Preparation of Alcohol-Insoluble Residue
The plant material (50 mg) was treated with 95% ethanol (1:4 w/v) at 100°C for 30 min. After the treatment, sample was centrifuged (10,000×g, 10 min), and the residue was subsequently washed five times with 70% ethanol and dried at 32°C under vacuum. The dried powder obtained after 70% ethanol wash is designated as alcohol-insoluble residue (AIR). The AIR was destarched essentially as described by Obro et al. [29]. AIR was incubated with heat-stable amylase from Bacillus licheniformis (Megazyme, Bray, Ireland) at 0.3 U per 10 mg AIR in 3-(N-morpholino) propanesulfonic acid buffer (50 mM, pH 7.0) at 85°C for 1 h. Subsequently, the sample was incubated with amyloglucosidase from Aspergillus niger (0.33 U per 10 mg AIR) and pullulanase from B. licheniformis (0.04 U/10 mg AIR) in 200 mM sodium acetate (pH 4.5), for 2 h at 50°C. Amyloglucosidase and pullulanase were purchased from Megazyme. The reaction was stopped by adding three volumes of 95% ethanol, vortexed and centrifuged at 10,000×g for 10 min. The residue obtained after centrifugation was washed ten times with 70% ethanol and dried at 32°C under vacuum. The destarched AIR was used as biomass in the pretreatment experiments.
Ionic Liquid Pretreatment of Biomass
Biomass (destarched AIR) was treated with 1-ethyl-3-methylimidazolium acetate (Sigma-Aldrich, St Louis, MO, USA) at a loading of 3% at 110–160°C for 3 h in an oven (Thelco Laboratory oven). Upon cellulose regeneration with water, the pretreated material was washed with deionized hot water. Samples were centrifuged at 10,000×g for 20–25 min, and washes were continued until a colorless supernatant was obtained to ensure complete washing of regenerated biomass. This indicated the absence of ionic liquid in wash which was further confirmed with Fourier transform infrared measurement. The infrared spectrum of supernatant showed no ionic liquid peaks. The pooled washes were concentrated under vacuum for further analysis. For residence time optimization, biomass at 3% loading was treated with 1-ethyl-3-methylimidazolium acetate. The temperature was varied from 110°C to 160°C at increments of 10°C for 3, 6, 24 h, 2, 3, 4, and 5 days in an oven. Pretreated material was washed and pooled as described above.
Monosaccharide Composition
After IL pretreatment and precipitation of cellulose by water, all the supernatants from the washing steps were collected and concentrated. Three-hundred microliters of solution was treated with 150 μl of trifluoroacetic acid (TFA) at 120°C for 1 h. The supernatant was placed in a CentriVap Vacuum Concentrator (Labconco Corp, Kansas City, MO, USA) at 32°C. Monosaccharides produced from untreated and pretreated samples both before and after TFA hydrolysis were analyzed by high-performance anion-exchange chromatography (HPAEC) on an ICS-3000 system equipped with an electrochemical detector and a 4 × 250 mm CarboPac PA™ 20 column (Dionex, Sunnyvale, CA, USA), according to Obro et al. [29]. The monosaccharides including fucose, arabinose, rhamnose, galactose, mannose, xylose, glucose, glucuronic acid, and galacturonic acid used as the external standards for HPAEC were obtained from Sigma-Aldrich and Alfa Aesar (Ward Hill, MA, USA).
Porosimetry of Biomass
Nitrogen porosimetry (Micromeritics ASAP 2020, Norcross, GA, USA) was used to measure the surface area, pore size distribution, and pore volume of the untreated and IL pretreated switchgrass. Samples were degassed at 100°C for 15 h and were cooled in liquid nitrogen, allowing nitrogen gas to condense on the surfaces and within the pores. Each data point along the isotherm was taken with a minimum equilibration time of 100 s to allow the pressure in the sample holder to stabilize. The quantity of gas that condensed could be inferred from the pressure decrease after the sample was exposed to the gas.
Lignin Quantification Using Acetyl Bromide
The lignin content of both untreated and regenerated biomass was determined with a modified acetyl bromide method [30, 31]. Switchgrass powder (5 mg) was treated with 25% (w/w) acetyl bromide in glacial acetic acid (0.2 ml). The tubes were sealed and incubated at 50°C for 2 h at 1,050 rpm on a thermomixer. After digestion, the solutions were diluted with three volumes of acetic acid, and then 0.1 ml was transferred to 15-ml centrifuge tubes and 0.5 ml acetic acid was added to it. The solutions were mixed well and 0.3 M sodium hydroxide (0.3 ml) and 0.5 M hydroxylamine hydrochloride (0.1 ml) were added to it. The final volume was made to 2 ml with the addition of acetic acid. The UV spectra of the solutions were measured against a blank prepared using the same method. The lignin content was determined with the absorbance at 280 nm and calculated with an averaged extinction coefficient of 18.1951 l g−1 cm−1 for grass samples [30]. The reagents used were from Alfa Aesar.
Enzymatic Saccharification
The untreated samples and regenerated biomass from various conditions were hydrolyzed in a batch system. The total batch volume was 10 ml of 50 mM sodium citrate buffer (pH 4.8) with 80 mg glucan contents, cellulase (cellulase from Trichoderma reesei, Worthington Biochemical Corporation, Lakewood, NJ, USA) with a loading of 12.5 IU/ml, and β-glucosidase (Novozyme 188, Novozymes Corporation, Davis, CA, USA) with a loading of 5.1 IU/ml. The digestion vials were incubated in a rotary shaker under the conditions of 150 rpm and 50°C. Experiments were conducted in triplicate for 72 h. The reaction was monitored by periodically taking evenly mixed slurry samples, centrifuging at 16,100×g for 10 min, and measuring the release of soluble reducing sugars by using 3,5-dinitrosalicylic acid (DNS, Sigma-Aldrich) colorimetric assay with d-glucose as a standard [32]. The supernatants (60 μl) were mixed with DNS solution (60 µl) and heated at 95°C for 5 min. After cooling down, their absorbances were taken at 540 nm [33]. The initial rates of formation of total soluble reducing sugars were calculated based on the sugar released in the first 30 min of hydrolysis [13].
Results and Discussion
Hemicellulose Disposition and Pattern of Sugar Release Using HPAEC
To understand the impact of IL pretreatment on polysaccharides and disposition of hemicellulose, process streams were analyzed and monitored by HPAEC. The results on the HPAEC profile of the supernatant for all the temperature series and residence times of IL pretreatment tested in the present study show only trace amounts of monosaccharides (Fig. 2a, b). This indicates that the IL did not result in complete depolymerization of hemicelluloses. IL pretreatment resulted in some depolymerization of hemicellulose into oligosaccharides which are observed at longer retention times but were not quantified in the current HPAEC system. To gain insight into the pattern of sugar release for various residence times and pretreatment temperatures, the supernatant was first digested with TFA and analyzed.
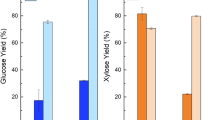
The HPAEC profile after TFA digestion (Fig. 3 and Table 1) shows the effect of residence time and temperature during IL pretreatment on the composition of supernatants. The residence time for pretreatment was varied from 3 h to 5 days at 120°C. As shown in Fig. 3, increased IL pretreatment time progressively led to increased oligosaccharide release. The major monosaccharides identified include xylose, arabinose, glucose, galactose, and rhamnose, whereas fucose, galacturonic acid, and glucuronic acid were present in trace amount. At 5 days, 0.074 µg xylose per microgram of biomass was released, which was four times higher than at 3 h and 1.3 times higher than after 24 h of pretreatment. A similar pattern was observed for release of arabinose, glucose, and galactose. The pretreatment temperature was varied from 110°C to 160°C for 3 h residence time. Table 1 illustrates that all sugar yields increased with the temperature increase following incubation for 3 h and pretreatment at 160°C removed a significant amount of hemicellulose, with the total saccharide yields 2–12 times higher than the corresponding values obtained for 110°C to 150°C pretreatments, indicating that IL pretreatment at higher temperature effectively disrupt the carbohydrate–lignin linkages and release hemicellulose. These results show that IL pretreatment is analogous to ammonia fiber expansion [22] pretreatment in terms of polysaccharide depolymerization to oligosaccharides. This is in contrast to acid pretreatment which is reported [10, 34] to release monomeric sugars upon pretreatment and is problematic since, at high pretreatment temperatures, the monomeric sugar may produce inhibitory complexes like furfural and HMF via ring opening of the xylose and glucose, respectively.
Effect of IL Pretreatment on Porosity and Surface Area of Biomass
The surface area was calculated using the Brunauer–Emmett–Teller (BET) model. This model was proposed and named for Brunauer, Emmett, and Teller who published their model in 1938 to propose a generalization to the Langmuir theory of monolayer adsorption [35]. The BET model relates the gas pressures and the volume of gas adsorbed according to the equation:
where p is the equilibrium pressure, p 0 is the saturation pressure, v is the volume of the adsorbed gas, v m is the volume of gas that would be required to cover all the surfaces with a monolayer, and c is the BET constant. The values of p, p 0, and v are measured directly during the experiment, and the values of v m and c can therefore be inferred by plotting \( \frac{p}{{v\left( {{p_0} - p} \right)}} \) against \( \frac{p}{{{p_0}}} \) and solving for v m and c from the slope \( \frac{{c - 1}}{{{v_{\rm{m}}}c}} \) and the intercept \( \frac{1}{{{v_{\rm{m}}}c}} \). The number of gas molecules that should theoretically cover a monolayer can be calculated from v m, and the BET surface area can be determined by multiplying by the molecular cross section of the adsorbate. For our experiments, the molecular cross section of nitrogen was assumed to be 0.1620 nm2 [35, 36].
Figure 4a, b compares the adsorption isotherms and pore size distributions, respectively, for switchgrass at two pretreatment temperatures with untreated samples. The difference between the untreated switchgrass and the switchgrass treated at 120°C is marginally noticeable but not nearly as dramatic as the difference between the untreated switchgrass and the switchgrass treated at 160°C. Figure 4a shows a significant increase in the quantity of gas adsorbed for the switchgrass treated at 160°C, indicating a higher specific surface area and a greater pore volume. There is a 30-fold increase in the BET surface area (15.8 vs 0.5 m2/g) between the switchgrass treated at 160°C and the untreated material [36]. The pore size and pore volume were calculated using the Barrett–Joyner–Halenda (BJH) method. The BJH method is named for Barrett, Joyner, and Halenda and is the classical model commonly used for determining pore size distributions [36]. In addition to the gas adsorption on the surfaces, additional gas can condense inside pores if the pressure is high enough. This critical pressure is determined from the Kelvin equation. The BJH method starts with the highest equilibration pressures that correspond to the largest pores. It then incrementally calculates the pore volume contained by pores with radii between the two critical radii corresponding to two adjacent equilibration pressures. Since additional gas condenses on the sidewalls, this “wall thickness” is also taken into account when determining the pore size distribution. Using this method, the volume of pores can be plotted as a function of pore radius, thereby producing a pore size distribution and a value for the total pore volume.
The pore size distributions are shown in Fig. 4b, and a quantitative summary of the measurements is shown in Fig. 4c.
Enzymatic Saccharification
Effect of Pretreatment Temperature
Figure 5a shows the total reducing sugar production and cellulose digestibility as a function of time during the enzymatic hydrolysis of untreated switchgrass and switchgrass pretreated for 3 h at temperatures ranging from 110°C to 160°C. Figure 5b further illustrates the initial rates of hydrolysis to soluble sugars. Taking into account the hydrolysis reaction stoichiometry, 1 g of cellulose upon complete hydrolysis produces 1.11 g of glucose [13]. When compared with untreated switchgrass, the IL pretreated switchgrass exhibited significantly faster cellulose to sugar conversion efficiency. Specifically, the amount of reducing sugars released during the first 3 h increased from 2.84 to 7.44 mg/ml with the temperature increase from 110°C to 160°C. After being hydrolyzed for 24 h, the reducing sugar obtained was 8.20 mg/ml for 160°C pretreated samples with the cellulose digestibility of 92.34%, whereas the sugar and digestibility were 6.75 mg/ml and 76.01% for switchgrass pretreated at 110°C and 0.31 mg/ml and 8.78% for untreated switchgrass, respectively. This implies that the pretreatment with IL at higher temperatures effectively increased the sugar recovery and cellulose digestibility. Correspondingly, as shown in Fig. 5b, the enzymatic kinetics of switchgrass pretreated at 160°C (0.18 mg ml−1 min−1) was 6.1 times higher than 110°C pretreated sample (0.03 mg ml−1 min−1) and up to 39 times higher than the untreated sample (0.0048 mg ml−1 min−1). After 48 h, most of the regenerated switchgrass was converted to soluble sugars and the solutions were clear, while a large fraction of the untreated switchgrass remained suspended in the mixture even after 48 h of digestion with cellulose cocktail, suggesting only partial hydrolysis.
Effect of Residence Time
In order to understand the effect of residence time of switchgrass samples in IL, the incubation time was varied from 3 h to 5 days. The regenerated switchgrass was then hydrolyzed in a batch system. Results show that the yield of reducing sugars (Fig. 6a) and the corresponding enzymatic kinetics (Fig. 6b) of switchgrass pretreated with IL at 120°C from 3 h to 5 days were similar, indicating that the pretreatment time has little effect on the enzymatic saccharification. However, pretreatment time has significant effect on the switchgrass pretreated at 160°C with the optimum condition at 3 h, whereas there was very little sugar production from the 24 h sample (Fig. 6c), with the yield even lower than the untreated switchgrass. This is likely due to the reaction of IL with cellulose and warrants further investigation.
Biomass Delignification
Lignin is the third major component of lignocellulosic biomass and provides a robust linkage between polysaccharide chains. The lignin seal and strong linkages obstruct enzyme accessibility to the polysaccharides [37] and can often irreversibly adsorb cellulases [38, 39], resulting in the need for high enzyme loading for digestion. In order to understand delignification during IL pretreatment, pretreated and regenerated biomass upon antisolvent addition was analyzed for lignin. Figure 7 shows the delignification achieved at different IL pretreatment temperatures. Several other studies also show the delignification during IL pretreatment. Sun et al. investigated the [C2mim][OAc] dissolution of pine and oak wood materials at 110°C for 16 h and achieved lignin reduction of 26.1% for pine and 34.9% for oak [15], which is significantly lower than the delignification efficiency of 73.5% for switchgrass at 160°C observed in this study. Lee et al. [38]used [C2mim][OAc] to extract the lignin from maple wood flour and achieved 85% of lignin after 70 h pretreatment at 90°C. In addition, 93% lignin extraction efficiency was reported from sugarcane bagasse using 1-ethyl-3-methylimidazolium alkyl benzene sulfonate at 190°C for 2 h [40]. The differences in the reported delignification efficiencies are likely due to two main reasons: First, the more effective pretreatments are generally those that employ higher temperatures and incubation times. This general trend shows an inverse relationship between pretreatment time and temperature, and the most efficient delignification temperature is strongly related to average glass transition temperature of 165°C for a given lignin polymer [41]. The actual glass transition temperature of the lignin is dictated by the chemical composition (monolignol composition and concentrations) and varies significantly between grasses, agricultural residues, softwoods, and hardwoods. Secondly, specific ILs have specific interactions with biomass, and those interactions are known to be dependent on the cation, anion, temperature, and time used in the pretreatment process. Correlation of enzymatic kinetics with lignin content (Fig. 7) indicates that increase in lignin removal efficiency is roughly paralleled with the acceleration of enzymatic hydrolysis rate, indicating a strong connection between delignification and enzymatic hydrolysis, which is consistent with the findings of Lee et al. [39]. These observations show that IL pretreatment results in significant level of delignification. In addition to quantification of total lignin amount by acetyl bromide method, our recent study also achieved 69.2% of total lignin removal efficiency with 12.0% of acid-soluble lignin and 57.2% of Klason lignin from switchgrass at 160°C [10]. The acetyl bromide method of lignin quantification was consistent with Klason lignin quantification by the NREL LAP procedure (both in house and by an external service Microbac Laboratory from Boulder, CO, USA), providing confidence in the adaptation of both methods for fairly nascent IL pretreatment technology.
Mass Balance for the Ionic Liquid Process
An analysis of the mass and composition of the untreated switchgrass and IL pretreatment products has been carried out to develop a proper mass balance for the IL pretreatment process of switchgrass conducted in this study. Figure 8 shows the mass balance for (a) untreated switchgrass and regenerated switchgrass from (b) 120°C and 3 h and (c) 160°C and 3 h pretreatment. In the untreated switchgrass, cellulose, hemicellulose, and lignin are the three major components accounting for 39%, 26%, and 23% total biomass, respectively, with the remaining 12% as structural inorganics, acetyl, and proteins (Fig. 8a). This result is consistent with microbac analysis carried out on the same batch of switchgrass. Process monitoring with HPAEC for total sugar released in the supernatant and for regenerated switchgrass by enzymatic hydrolysis (total reducing sugar yields) provides understanding of the fraction of solid and liquid streams for polysaccharides (cellulose and hemicelluloses). Results show that IL pretreatment at 160°C released only 8% (20.5% of original glucan amount in untreated biomass) of glucan into the supernatant, and regenerated solid is composed of 31% of glucan (80% of the original glucan). In contrast, the majority of xylan (19%) ends up in the liquid stream upon IL pretreatment which is 73% of the original amount in the untreated switchgrass. For IL pretreatment conducted at 160°C (highest temperature condition possible for [C2mim] [OAc] since this IL is thermally unstable at >165°C), 17% lignin (out of a total of 23% lignin in the starting material) was detected in the liquid stream, and the recovered solid (pretreated and regenerated switchgrass) was composed of only 5% lignin. In comparison, ionic pretreatment at 120°C only removed 5% of xylan and 9% of lignin, and 56% of the native carbohydrates in the original switchgrass were regenerated in the cellulose-rich material along with 17% of the native lignin still bonded. The process monitoring and mass balance suggest very little loss of material during handling and IL pretreatment. Detailed studies of the specific composition of the lignin in the supernatant and recovered biomass will provide better insight of the nature of lignin recalcitrance in addition to lignin disposition carried out in present studies.
Conclusions
Detailed parametric studies of IL pretreatment of switchgrass have been carried out to understand the disposition of cellulose, hemicellulose, and lignin in order to optimize the [C2mim][OAc] pretreatment process. Our findings indicate that efficient depolymerization of hemicellulose occurs regardless of residence time or temperature. The hemicellulose is converted to oligosaccharides, and only trace amounts of monomeric sugars (xylose and glucose) are detected in the IL hydrolysates. Quantification of xylose, glucose, arabinose, galactose, rhamnose, and other minor C6 and C5 sugars after TFA digestion of IL-pretreated and regenerated biomass as a function of temperature and residence time shows very different patterns of sugar release. Temperature studies show that three times as much oligomeric hemicellulose are released at 160°C when compared to 120°C. IL pretreatments conducted at 100–140°C show similar total sugar yields in the IL hydrolysate supernatant.
Three hour IL pretreatment delignified switchgrass by 73.5% at 160°C. An interesting observation was significant enhancement in delignification at 150°C. This is consistent with the reported process temperatures of acid and ammonia fiber expansion pretreatment technologies. These results suggest softening or melting of lignin to be primarily responsible for observed increase in enzymatic hydrolysis kinetics of pretreated biomass. In addition, the temperature variation of IL pretreatment from 110°C to 160°C resulted in lignin removal efficiency that is monotonically related to the increase of enzymatic hydrolysis.
It is also observed that the BET surface area increased ∼30-fold after IL treatment at 160°C. Pore volume (BJH absorption) also increased ∼30-fold after IL pretreatment with average measured pore size of 10–15 nm for [C2mim][OAc]-pretreated switchgrass. Porosimetry data of untreated switchgrass indicate a nonporous matrix with minimal surface accessibility for cellulolytic enzymes.
Time-series experiments show that, for 120°C pretreatment, 3 h IL pretreatment is sufficient since 3 h and 5 day IL pretreatment show no difference in sugar yields. However, pretreatment residence time has a significant effect on switchgrass pretreated at 160°C with the optimum residence time found to be 3 h, whereas there was very little sugar production for the 24 h sample with the yield even lower than the untreated switchgrass. The biomass was completely solubilized for 160°C pretreated sample with a residence time of 5 days and resulted in no recovery of biomass. It is interesting that 160°C and 5 day IL pretreatment resulted in IL hydrolysate which showed presence of oligosaccharides and only trace amounts of monosaccharides by HPAEC measurements. Saccharification kinetics were ∼3.8 times faster for 160°C pretreated switchgrass than 120°C pretreated switchgrass. However, at 160°C, the hydrolysis kinetics increased ∼6.1 times when compared to 110°C pretreatment showing doubling of kinetics for 10°C increase in pretreatment temperature and reached to a maximum of ∼39 times higher than the untreated switchgrass. This observed enhancement of enzymatic hydrolysis kinetics is significant and substantiates the importance of pretreatment technologies for rapid advancement of biofuel production from lignocellulosic biomass.
References
Simmons BA, Loque D, Blanch HW (2008) Next-generation biomass feedstocks for biofuel production. Genome Biol 9(12):242
Blanch HW, Wilke CR (1982) Sugars and chemicals from cellulose. Rev Chem Eng 1:71–119
Ralph J, Grabber JH, Hatfield RD (1995) Lignin-ferulate cross-links in grasses—active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydr Res 275(1):167–178
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioproducts Biorefining 2(1):26–40
Zhang YHP, Ding SY, Mielenz JR, Cui JB, Elander RT, Laser M et al (2007) Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol Bioeng 97(2):214–223
Bochek AM, Kalyuzhnaya LM (2002) Interaction of water with cellulose and cellulose acetates as influenced by the hydrogen bond system and hydrophilic–hydrophobic balance of the macromolecules. Russ J Appl Chem 75(6):989–993
Bochek AM (2003) Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ J Appl Chem 76(11):1711–1719
Lloyd TA, Wyman CE (2005) Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol 96(18):1967–1977
Lau MW, Dale BE, Balan V (2008) Ethanolic fermentation of hydrolysates from ammonia fiber expansion (AFEX) treated corn stover and distillers grain without detoxification and external nutrient supplementation. Biotechnol Bioeng 99(3):529–539
Li C, Knierim B, Manisseri C, Arora R, Scheller HV, Auer M et al (2009) Comparison of dilute acid and ionic liquid pretreatment of switchgrass: biomass recalcitrance, delignification and enzymatic saccharification. BioresourTechnol. doi:10.1016/j.biortech.2009.10.066
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2003) Ionic liquids as green solvents for the dissolution and regeneration of cellulose. Abstr Pap Am Chem Soc 225:U288–U288
Singh S, Simmons BA, Vogel KP (2009) Visualization of biomass solubilization and cellulose regeneration during ionic liquid pretreatment of switchgrass. Biotechnol Bioeng 104(1):68–75
Dadi AP, Varanasi S, Schall CA (2006) Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol Bioeng 95(5):904–910
Dadi AP, Schall CA, Varanasi S (2007) Mitigation of cellulose recalcitrance to enzymatic hydrolysis by ionic liquid pretreatment. Appl Biochem Biotechnol 137:407–421
Sun N, Rahman M, Qin Y, Maxim ML, Rodriguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chemistry 11(5):646–655
Zhao H, Jones CIL, Baker GA, Xia S, Olubajo O, Person VN (2009) Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J Biotechnol 139(1):47–54
Kumar R, Wyman CE (2008) The impact of dilute sulfuric acid on the selectivity of xylooligomer depolymerization to monomers. Carbohydr Res 343(2):290–300
Chen SF, Mowery RA, Chambliss CK, van Walsum GP (2007) Pseudo reaction kinetics of organic degradation products in dilute-acid-catalyzed corn stover pretreatment hydrolysates. Biotechnol Bioeng 98(6):1135–1145
Ramos LP (2003) The chemistry involved in the steam treatment of lignocellulosic materials. Quim Nova 26(6):863–871
Stoll M, Fengel D (1986) Cellulose crystals in trifluoroacetic-acid (TFE) solutions. Holz Roh Werkst 44(10):394–394
Mosier N, Hendrickson R, Ho N, Sedlak M, Ladisch MR (2005) Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour Technol 96(18):1986–1993
Kumar R, Mago G, Balan V, Wyman CE (2009) Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour Technol 100(17):3948–3962
Kosan B, Michels C, Meister F (2008) Dissolution and forming of cellulose with ionic liquids. Cellulose 15(1):59–66
McLaughlin SB, Kszos LA (2005) Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenergy 28(6):515–535
Sarath G, Baird LM, Vogel KP, Mitchell RB (2007) Internode structure and cell wall composition in maturing tillers of switchgrass (Panicum virgatum L.). Bioresour Technol 98(16):2985–2992
Schmer MR, Vogel KP, Mitchell RB, Moser LE, Eskridge KM, Perrin RK (2006) Establishment stand thresholds for switchgrass grown as a bioenergy crop. Crop Sci 46(1):157–161
Schmer MR, Vogel KP, Mitchell RB, Perrin RK (2008) Net energy of cellulosic ethanol from switchgrass. Proc Natl Acad Sci U S A 105(2):464–469
Boylan D, Bush V, Bransby DI (2000) Switchgrass cofiring: pilot scale and field evaluation. Biomass Bioenergy 19(6):411–417
Obro J, Harholt J, Scheller HV, Orfila C (2004) Rhamnogalacturonan I in Solanum tuberosum tubers contains complex arabinogalactan structures. Phytochemistry 65(10):1429–1438
Fukushima RS, Hatfield RD (2004) Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J Agric Food Chem 52(12):3713–3720
Pandey KK, Pitman AJ (2004) Examination of the lignin content in a softwood and a hardwood decayed by a brown-rot fungus with the acetyl bromide method and Fourier transform infrared spectroscopy. J Polym Sci A Polym Chem 42(10):2340–2346
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Xiao Z, Storms R, Tsang A (2005) Microplate-based carboxymethyl cellulose assay for endoglucanase activity. Anal Biochem 342:176–178
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY et al (2009) Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol Prog 25(2):333–339
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. 1. Computations from nitrogen isotherms. J Am Chem Soc 73(1):373–380
Zhu Z, Sathitsuksanoh N, Vinzant T, Schell DJ, McMillan JD, Zhang YH (2009) Comparative study of corn stover pretreated by dilute acid and cellulose solvent-based lignocellulose fractionation: enzymatic hydrolysis, supramolecular structure, and substrate accessibility. Biotechnol Bioeng 103(4):715–724
Ooshima H, Sakata M, Harano Y (1986) Enhancement of enzymatic-hydrolysis of cellulose by surfactant. Biotechnol Bioeng 28(11):1727–1734
Lee SH, Doherty TV, Linhardt RJ, Dordick JS (2009) Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol Bioeng 102(5):1368–1376
Tan SSY, MacFarlane DR, Upfal J, Edye LA, Doherty WOS, Patti AF et al (2009) Extraction of lignin from lignocellulose at atmospheric pressure using alkyl benzene sulfonate ionic liquid. Green Chem 11(3):339–345
Mikiji Shigematsu (1994) Enhancement of miscibility between hemicellulose and lignin by addition of their copolymer, the lignin–carbohydrate complex. Macromol Chem Phys 195(8):2827–2837
Acknowledgments
This work was part of the DOE Joint BioEnergy Institute (http://www.jbei.org) supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US Department of Energy. The authors thank Drs. Patanjali Varanasi and Anthe George from the Joint BioEnergy Institute for their help with manuscript proofreading and their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arora, R., Manisseri, C., Li, C. et al. Monitoring and Analyzing Process Streams Towards Understanding Ionic Liquid Pretreatment of Switchgrass (Panicum virgatum L.). Bioenerg. Res. 3, 134–145 (2010). https://doi.org/10.1007/s12155-010-9087-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-010-9087-1