Abstract
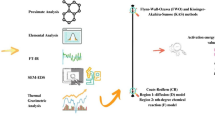
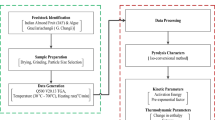
This study was conducted to investigate the kinetic, thermodynamics and the reaction mechanism of pyrolysis of native walnut shells of Kashmir, India. Thermal degradation experiments were performed at three heating rates of 10, 25, and 50 K min−1 to calculate the kinetic and thermodynamic parameters, using iso-conversional Kissinger-Akahira-Sunrose (KAS) and Ozawa-Flynn-Wall (OFW) models. The reaction mechanism was predicted by applying Coats-Redfern (CR) method. Moreover, an artificial neural network (ANN) simulation was used to obtain best fit points after comparing the experimental data with the predicted data points. Average activation energy was calculated from the thermogravimetric study was found to be in the range of 146.03–148.89 kJ mol−1, while the Gibbs free energy (ΔG) value for walnut shells was found to be ~180 kJ mol−1. The most appropriate degradation mechanism was found to be based on diffusion and chemical reaction for the temperature range under study. The broad characterisation along with the values of thermodynamic parameters show that the walnut shells can be used as an economical as well as eco-friendly bio-energy feed-stock for pyrolysis. The reaction mechanism of thermal degradation of walnut shells was found to be consisting of two broader zones based on conversion achieved, zone I (0.2 ≤ α ≤ 0.4) and zone II (0.4 ≤ α ≤ 0.8).





Similar content being viewed by others
Abbreviations
- A o :
-
Pre-exponential factor, s−1
- ANN:
-
Artificial neural network
- CR:
-
Coats-Redfern method
- D1–5:
-
Diffusion-based mechanisms
- DTA:
-
Differential thermal analysis
- DTG:
-
Differential thermogravimetry
- E t :
-
Activation energy, kJ mol−1
- F1–5:
-
Chemical reaction-based mechanism
- HHV:
-
High heating value, MJ g−1
- k (T):
-
Reaction rate constant
- K b :
-
Boltzman constant, 1.381 × 10−23 J K−1
- KAS:
-
Kissinger-Akahira-Sunrose
- MSE:
-
Mean square error
- OFW:
-
Ozawa-Flynn-Wall method
- o :
-
Output values
- R1–5:
-
Contacting geometry-based mechanism
- t :
-
Target values
- α :
-
Conversion
- ∆G :
-
Gibbs free energy, kJ mol−1
- ∆H :
-
Change in enthalpy, kJ mol−1
References
Abnisa F, Arami-Niya A, Daud WMAW, Sahu JN, Noor IM (2013) Utilization of oil palm tree residues to produce bio-oil and bio-char via pyrolysis. Energy Convers Manag 76:1073–1082
Ningbo G, Baoling L, Aimin L, Juanjuan L (2015) Continuous pyrolysis of pine sawdust at different pyrolysis temperatures and solid residence times. J Anal App Pyrol 114:155–162
Mukherjee A, Das P, Minu K (2014) Thermogravimetric analysis and kinetic modelling studies of selected agro-residues and biodiesel industry wastes for pyrolytic conversion to bio-oil. Biomass Conv Bioref 4:259–258
Pradhan RR, Garnaik PP, Regmi B, Dash B, Dutta A (2017) Pyrolysis kinetics of sal (Shorea robusta) seeds. Biomass Conv Bioref 7:237–247
Acikalm K (2011) Thermogravimetric analysis of walnut shell as pyrolysis feedstock. J Therm Anal Calorim 105:145–150
Demirbas A (2006) Effect of temperature on pyrolysis products from four nut shells. J Anal Appl Pyrol 76:285–289
Yuan HR, Liu RH (2007) Study on pyrolysis kinetics of walnut shell. J Therm Anal Calorim 89-3:983–986
Cai JM, Wu WX, Liu RH, Huber GW (2013) A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem 15:1331–1340
Wu W, Mei Y, Zhang L, Liu R, Cai J (2014) Effective activation energies of lignocellulosic biomass pyrolysis. Energ Fuel 28:3916–3923
Irdem SD, Parparita E, Vasile C, Uddin MA, Yanik J (2014) Steam reforming of tar derived from walnut shell and almond shell gasification on red mud and iron-ceria catalysts. Energ Fuel 28:3808–3813
Kalogirou SA (2003) Artificial intelligence for the modeling and control of combustion processes: a review. Prog Energy Combust Sci 29:515–566
Ahmad MS, Mehmood MA, Taqvi STH, Elkamel A, Liu C, Xu J, Rahimuddin SA, Gull M (2017) Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour Technol 245:491–501
Yıldız Z, Uzun H, Ceylan S, Topcu Y (2016) Application of artificial neural networks to co-combustion of hazelnut husk–lignite coal blends. Bioresour Technol 200:42–47
Sharma RM, Kour K, Singh B, Yadav S, Kotwal N, Rana JC, Anand R (2014) Selection and characterization of elite walnut (Juglans regia L.) clone from seedling origin trees in North Western Himalayan region of India. Aust J Crop Sci 8(2):257–262
Sharma R (2012) Area and production database of fruit crops. Directorate of hortic state department of horticulture. Jammu and Kashmir government, Srinagar, India
Uzun BB, Yaman E (2014) Thermogravemetric characteristics and kinetics of scrap tyre and juglans regia shell co-pyrolysis. Waste Manage Res 32(10):961–970
Akahira T, Sunrose T (1969) Transactions of Joint Convention of Four Electrical Institutes. 246
Flynn JH, Wall LA (1966) A quick, direct method for the determination of activation energy from thermogravimetric Data. J Polym Sci Pol Lett 5:323–328
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Japan 38:1881–1886
Chen D, Zhou J, Zhang Q (2014) Effects of torrefaction on the pyrolysis behaviour and bio-oil properties of rice husk by using TG-FTIR and Py-GC/MS. Energ Fuel 28:5857–5863
Doyle CD (1965) Series approximations to the equations of thermo-gravimetric data. Nature 207:290–291
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A (2011) Thermal degradation studies and kinetic modelling of cardoon (Cynaracardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol 102(10):6230–6238
Ceylan S, Topcu Y (2014) Pyrolysis kinetics of hazelnut husk using thermo-gravimetric analysis. Bioresour Technol 156:182–188
Mythili R, Venkatachalam P, Subramanian P, Uma D (2013) Characterization of bio residues for bio oil production through pyrolysis. Biresourc Technol 138:71–78
Toshniwal P, Srivastava VC (2017) Existence of synergistic effects during co-pyrolysis of petroleum coke and wood pellet. Intl J Chem React Eng 20160046:1–12
El-Sayed SA, Mostafa ME (2015) Kinetic parameters determination of biomass pyrolysis fuels using TGA and DTA Techniques. Waste Biomass Valori 6:401–415
Ferreira CIA, Calisto V, Cuerda-Correa EM, Otero M, Nadais H, Esteves VI (2016) Comparative valorisation of agricultural and industrial bio wastes by combustion and pyrolysis. Bioresour Technol 218:918–925
Kilic M, Kirbiyik C, Cepeliogullar O, Putun AE (2013) Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl Surf Sci 283:856–862
Dandekar A, Baker RTK, Vannice MA (1998) Characterization of activated carbon, graphitized carbon fibres and synthetic diamond power using TPD and DRIFTS. Carbon 36:1821–1831
Hanafiah MAKM, Ngah WSW, Zolkafly SH, Teong LC, Majid ZA (2012) Acid Blue 25 adsorption on base treated Shorea dasyphylla sawdust: Kinetic, isotherm, thermodynamic and spectroscopic analysis. J Environ Sci 24:261–268
Yang J, Fang F, Zhou J (2013) Effect of microstructure and surface chemistry in liquid-phase adsorptive nicotine by almond-shell-based activated carbon. Chinese Sci Bull 58(30):3715–3720
Grube M, Lin J, Lee P, Kokorevicha S (2006) Evaluation of sewage sludge-based compost by FT-IR spectroscopy. Geoderma 130:324–333
Mehmood MA, Ye G, Luo H, Liu C, Malik S, Afzal I, Xu J, Ahmad MS (2017) Pyrolysis and kinetic analyses of camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour Technol 228:18–24
Braga RM, Melo DM, Aquino FM, Freitas JC, Melo MA, Barros JM, Fontes MS (2014) Characterization and comparative study of pyrolysis kinetics of the rice husk and the elephant grass. J Therm Anal Calorim 115(2):1915–1920
Kim YS, Kim YS, Kim SH (2010) Investigation of thermodynamic parameters in the thermal decomposition of plastic waste–waste lube oil compounds. Environ Sci Technol 44:5313–5317
Xu Y, Chen B (2013) Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresource Technol 146:485–493
Vlaev L, Georgieva V, Genieva S (2007) Products and kinetics of non-isothermal decomposition of vanadium (IV) oxide compounds. J Therm Anal Calorim 88:805–812
Turmanova SC, Genieva S, Dimitrova A, Vlaev L (2008) Non-isothermal degradationkinetics of filled with rise husk ash polypropene composites. Express Polym Lett 2:133–146
Maia AAD, de Morais LC (2016) Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour Technol 204:157–163
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110:17315–17328
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasool, T., Srivastava, V.C. & Khan, M.N.S. Utilisation of a waste biomass, walnut shells, to produce bio-products via pyrolysis: investigation using ISO-conversional and neural network methods. Biomass Conv. Bioref. 8, 647–657 (2018). https://doi.org/10.1007/s13399-018-0311-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0311-0