Abstract
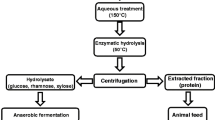
The chemical profile of biorefined Saccharina latissima, Ascophyllum nodosum and Palmaria palmata after carbohydrate and polyphenol extraction was analysed with the aim to evaluate the nutritional aspects of biorefined seaweeds as a novel animal feed supplement. Optimised enzymatic saccharification has been used to show that the protein concentration in the residue of P. palmata and A. nodosum can be increased by more than 2-fold. Nutritional value of the residue was further enhanced through an increase in total amino acids and fatty acids. As a consequence of removal of inorganic elements such as sodium, potassium and chloride, the total solid and ash content of all three seaweeds was reduced by around 40%. In contrast, divalent metals such as iron and zinc, as well as silicon, accumulated in all three residues. Potentially harmful components such as arsenic and iodine were reduced only in brown biorefined seaweeds, whilst in biorefined P. palmata, iodine increased by 39% compared to a 24% decline of arsenic. Nutritional values such as total fatty acid and total amino acid content increased in all three seaweeds after enzymatic saccharification. Polyphenol removal in all three seaweeds was >80% using aqueous acetonitrile and, in combination with enzymatic saccharification, did not impact on protein recovery in A. nodosum. This highlights the potential of biorefinery concepts to generate multiple products from seaweed such as extracts enriched in polyphenols and carbohydrates and residue with higher protein and lipid content.
Similar content being viewed by others
References
World Health Organization (2009) Global and regional food consumption patterns and trends. WHO, 1–17, Technical Report Series 916
Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Aiking H (2011) Future protein supply. Trends Food Sci Technol 22:112–120
Percival E (1979) The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Brit Phycol J 14:103–117
Gilani GS, Xiao CW, Cockell KA (2012) Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr 108:S315–S332
Amano H, Noda H (1992) Proteins of protoplasts from several seaweeds. Nippon Suisan Gakkaishi 58:291–299
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
van den Burg S, Stuiver M, Veenstra F, Bikker P, Contreras AL, Palstra A (2013) A triple P review of the feasibility of sustainable offshore seaweed production in the North Sea. Wageningen UR, Wageningen
McHugh DJ (2003) A guide to the seaweed industry. Food and Agriculture Organization of the United Nations, Rome
Montgomery WL, Gerking SD (1980) Marine macroalgae as foods for fishes: an evaluation of potential food quality. Environ Biol Fish 5:143–153
McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15:513–524
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Wang Y, McAllister A (2011) Brown algae as a feed additive: nutritional and health impacts on ruminant—a review. In: Borgearo SR (ed) Animal feed: types. Nova Science Publishers, Nutrition and Safety, pp. 1–32
Soler-Vila A, Coughlan S, Guiry MD, Kraan S (2009) The red alga Porphyra dioica as a fish-feed ingredient for rainbow trout (Oncorhynchus mykiss): effects on growth, feed efficiency, and carcass composition. J Appl Phycol 21:617–624
Siriwardhana N, Lee K, Kim S, Ha J, Park G, Jeon Y (2004) Lipid peroxidation inhibitory effects of Hizikia fusiformis methanolic extract on fish oil and linoleic acid. Food Sci Technol Int 10:65–72
R. B. Gravett (2000) The effects of Ascophylum nodosom on immune function, performance, and carcass characteristics of sheep and cattle. Dissertation, Texas Tech University
Evans F, Critchley A (2014) Seaweeds for animal production use. J Appl Phycol 26:891–899
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology. Cambridge University Press
de Jong E, Higson A, Walsh P, Wellisch M (2012) Bio-based chemicals value added products from biorefineries. IEA Bioenergy, Task 42 Biorefinery
Li K, Liu S, Liu X (2014) An overview of algae bioethanol production. Int J Energy Res 38:965–977
Schiener P, Atack T, Wareing R, Kelly MS, Hughes AD (2015) The by-products from marine biofuels as a feed source for the aquaculture industry: a novel example of the biorefinery approach. Biomass Conversion and Biorefinery 1–7
Bikker P, Krimpen MM, Wikselaar P, Houweling-Tan B, Scaccia N, Hal JW, Huijgen WJ, Cone JW, López-Contreras AM (2016) Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J Appl Phycol 1–15
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of ash in biomass. Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory, Golden, NREL/TP-510-42622
Schiener P, Black KD, Stanley MS, Green DH (2015) The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J Appl Phycol 27:363–373
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory, Colorado, Report N.TP-510-42618
Haug A, Melsom S, Omang S (1974) Estimation of heavy metal pollution in two Norwegian fjord areas by analysis of the brown alga Ascophylum nodosum. Environ Pollut 7:179–192
Murphy B, Dorich B, Richter B (2012) Extraction of total fat from food samples after acid hydrolysis using accelerated solvent extraction with GC-MS analysis. Thermo Fisher Scientific, Application Note 361
Henderson J, Brooks A (2010) Improved amino acid methods using Agilent ZORBAX Eclipse Plus C18 columns for a variety of Agilent LC instrumentation and separation goals. Agilent Technologies, Wilmington
Lahaye M, Vigouroux J (1992) Liquefaction of dulse (Palmaria palmata (L.) Kuntze) by a commercial enzyme preparation and a purified endo, β-1, 4-D-xylanase. J Appl Phycol 4:329–337
Khotimchenko YS, Khozhaenko EV, Khotimchenko MY, Kolenchenko EA, Kovalev VV (2010) Carrageenans as a new source of drugs with metal binding properties. Marine drugs 8:1106–1121
Haug A (1961) Affinity of some divalent metals to different types of alginates. Acta Chem Scand 15:1794
Zava TT, Zava DT (2011) Assessment of Japanese iodine intake based on seaweed consumption in Japan: a literature-based analysis. Thyroid Res 4:1–14
Hou X, Chai C, Qian Q, Yan X, Fan X (1997) Determination of chemical species of iodine in some seaweeds (I). Sci Total Environ 204:215–221
Teas J, Pino S, Critchley A, Braverman LE (2004) Variability of iodine content in common commercially available edible seaweeds. Thyroid 14:836–841
Rose M, Lewis J, Langford N, Baxter M, Origgi S, Barber M, MacBain H, Thomas K (2007) Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol 45:1263–1267
Nielsen MM, Manns D, D’Este M, Krause-Jensen D, Rasmussen MB, Larsen MM, Alvarado-Morales M, Angelidaki I, Bruhn A (2016) Variation in biochemical composition of Saccharina latissima and Laminaria digitata along an estuarine salinity gradient in inner Danish waters. Algal Res 13:235–245
Lardy GP (2002) Feeding corn to beef cattle. NDSU Extension Service
McDougall GJ, Stewart D (2005) The inhibitory effects of berry polyphenols on digestive enzymes. Biofactors 23:189–195
Nwosu F, Morris J, Lund VA, Stewart D, Ross HA, McDougall GJ (2011) Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem 126:1006–1012
Liu L, Su H, Yan S, Shao S, Xie B, Chen X, Zhang X, Zhou B, Zhang Y (2009) Probing the pH sensitivity of R-phycoerythrin: investigations of active conformational and functional variation. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1787:939–946
Cˇerná M (2011) Seaweed proteins and amino acids as nutraceuticals. Adv Food Nutr Res 64:297–312
Moroney NC, Wan AH, Soler-Vila A, FitzGerald RD, Johnson MP, Kerry JP (2015) Inclusion of Palmaria palmata (red seaweed) in Atlantic salmon diets: effects on the quality, shelf-life parameters and sensory properties of fresh and cooked salmon fillets. J Sci Food Agric 95:897–905
Cook EJ, Kelly MS (2007) Effect of variation in the protein value of the red macroalga Palmaria palmata on the feeding, growth and gonad composition of the sea urchins Psammechinus miliaris and Paracentrotus lividus (Echinodermata). Aquaculture 270:207–217
Marrion O, Schwertz A, Fleurence J, Gueant JL, Villaume C (2003) Improvement of the digestibility of the proteins of the red alga Palmaria palmata by physical processes and fermentation. Food/Nahrung 47:339–344
Fleurence J (1999) The enzymatic degradation of algal cell walls: a useful approach for improving protein accessibility? J Appl Phycol 11:313–314
MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR (2007) Nutritional value of edible seaweeds. Nutr Rev-Wash 65:535
Lahaye M, Rondeau-Mouro C, Deniaud E, Buléon A (2003) Solid-state 13 C NMR spectroscopy studies of xylans in the cell wall of Palmaria palmata (L. Kuntze, Rhodophyta). Carbohydr Res 338:1559–1569
Kraan S (2012) Algal polysaccharides, novel applications and outlook. INTECH Open Access Publisher
de Jesus R, de Maria Filomena M, de Alcina Maria Miranda Bernardo M, Rui Manuel Santos C (2016) Emergent sources of prebiotics: seaweeds and microalgae. Mar Drugs 14:27
Sharma S, Horn SJ (2016) Enzymatic saccharification of brown seaweed for production of fermentable sugars. Bioresour Technol
Manns D, Nyffenegger C, Saake B, Meyer AS (2016) Impact of different alginate lyases on combined cellulase–lyase saccharification of brown seaweed. RSC Adv 6:45392–45401
Percival E (1978) Do the polysaccharides of brown and red seaweeds ignore taxonomy. Modern Approaches to the Taxonomy of Red and Brown Algae. Systematics Assoc. Sp 10: 47–62
Mišurcová L, Škrovánková S, Samek D, Ambrožová J, Machu L (2012) 3. Health benefits of algal polysaccharides in human nutrition. Adv Food Nutr Res 66:75
El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B (2013) The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11:497–504
Hehemann J, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G (2010) Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 464:908–912
Hansen H, Hector B, Feldmann J (2003) A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim Feed Sci Technol 105:21–28
Li B, Lu F, Wei X, Zhao R (2008) Fucoidan: structure and bioactivity. Molecules 13:1671–1695
Tamminga S (1996) A review on environmental impacts of nutritional strategies in ruminants. J Anim Sci 74:3112–3124
Moen E, Horn S, Østgaard K (1997) Biological degradation of Ascophylum nodosum. J Appl Phycol 9:347–357
Barwell CJ, Blunden G, Manandhar PD (1989) Isolation and characterization of brown algal polyphenols as inhibitors of α-amylase, lipase and trypsin. J Appl Phycol 1:319–323
McAllan A, Theodorou M, Beever D (1988) Phenolics in fibrous crop residues and plants and their effects on the digestion and utilisation of carbohydrates and proteins in ruminants. Workshop on Plant Breeding and the Nutritive Value of Crop Residues, Addis Ababa (Ethiopia), 7–10 Dec 1987
Bandyopadhyay P, Ghosh AK, Ghosh C (2012) Recent developments on polyphenol–protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system. Food Function 3:592–605
Ozdal T, Capanoglu E, Altay F (2013) A review on protein–phenolic interactions and associated changes. Food Res Int 51:954–970
Bogolitsyn K, Kaplitsin P, Pochtovalova A (2014) Amino-acid composition of Arctic Brown algae. Chem Nat Compd 49:1110–1113
Galland-Irmouli A, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki J, Villaume C, Guéant J (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10:353–359
Zhou AY (2012) Protein content and amino acid profile in New Zealand Undaria pinnatifida. Dissertation, Auckland University of Technology
Cao Y, Duan J, Guo J, Guo S, Zhao J (2014) Rapid determination of nucleosides, nucleobases and free amino acids in brown seaweeds using ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry. J Appl Phycol 26:675–686
Mouritsen OG, Dawczynski C, Duelund L, Jahreis G, Vetter W, Schröder M (2013) On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). J Appl Phycol 25:1777–1791
Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, Wang B, Wang J, Yin Y (2013) Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 44:1107–1113
McLarney M, Pellett P, Young V (1996) Pattern of amino acid requirements in humans: an interspecies comparison using published amino acid requirement recommendations. J Nutr 126:1871
van Ginneken VJ, Helsper J, de Visser W, van Keulen H, Brandenburg WA (2011) Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids Health Dis 10:104
Gosch BJ, Magnusson M, Paul NA, Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy 4:919–930
Acknowledgements
This work was carried out as part of the EnAlgae project, which has received funding from the European Regional Development Funding via the INTERREG IVB NWE programme. The authors from Queen’s University of Belfast would like to acknowledge the support from Targeted Match Funding. A special thanks to three members of staff at Queen’s University of Belfast—Mrs. Emma Gorman, Prof. Andy Meharg and Mr. Philip McCarron—for their support leading up to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schiener, P., Zhao, S., Theodoridou, K. et al. The nutritional aspects of biorefined Saccharina latissima, Ascophyllum nodosum and Palmaria palmata . Biomass Conv. Bioref. 7, 221–235 (2017). https://doi.org/10.1007/s13399-016-0227-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-016-0227-5