Abstract
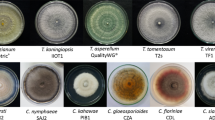
Trichothecium roseum causes the pink mold rot in many fruits and vegetables around the world. Due to this infection, significant losses arise in foods. In order to control this infection, plant extracts offer alternative treatment for fungicides. In this study, 50 plant species were screened for their antifungal effects against T. roseum. Anthemis arvensis, Origanum vulgare, Sambucus ebulus and Thymus longicaulis powders totally inhibited the mycelia growth of T. roseum at 10% (w/v). The powders of Chelidonium majus and Clinopodium vulgare were effective to T. roseum, with a percentage of inhibition of mycelia growth higher than 70%. MIC of A. arvensis aqueous extracts were lower than the other extracts (125 \(\upmu \hbox {g}\)/ml). Also its extracts inhibited the spore germination by 100% at 1000 \(\upmu \hbox {g}\)/ml. The incidence of the pink mold rot on tomatoes which were treated with C. majus aqueous extracts (75, 150 and 300 mg/ml) was lower than the extracts of other plants when compared to control. At concentration of 300 mg/ml, C. majus extracts prevented the disease by 71.42%. By the SEM, it was determined at the 4MIC extracts, cell wall degradation, swelling, flattening, lysis, collapsing and wrinkling on the hyphal structure. The highest total phenolic and flavanol contents were observed in O. vulgare extracts (310.49 mg GA/g) and T. longicaulis (5.24 mg CE/g). The \(\hbox {EC}_{50}\) values of the experimented extracts were lowered than the \(\hbox {EC}_{50}\) value of Gallic acid (1.87 mg/ml). Meanwhile, in all of the extracts there were phenolic compounds, protocatechuic, chlorogenic, caffeic acid and kaempferol as determined with HPLC system. This research demonstrates that C. majus aqueous extracts may possess high potential to control the pink mold rot on tomatoes as new natural antifungal products.
Similar content being viewed by others
References
Ameziane, N.; Boubaker, H.; Boudyach, H.; Msanda, F.; Jilal, A.; Benaoumar, A.A.: Antifungal activity of moroccan plants against citrus fruit pathogens. Agron. Sustain. Dev. 27, 273–277 (2007)
Phillips, C.A.; Laird, K.; Allen, S.C.: The use of Citri-VTM\(\textregistered \)—an antimicrobial citrus essential oil vapour for the control of Penicillium chrysogenum, Aspergillus niger and Alternaria alternata in vitro and on food. Food Res. Int. 47, 310–314 (2012)
Mishra, A.K.; Dubey, N.K.: Evaluation of some essential oils for their toxicity against fungi causing deterioration of stored food commodities. Appl. Environ. Microbiol. 60, 1101–1105 (1994)
Prakash, B.; Singh, P.; Kedia, A.; Dubey, N.K.: Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Res. Int. 49, 201–208 (2012)
Chen, F.; Long, X.; Yu, M.; Liu, Z.; Liu, L.: Phenolics and antifungal activities analysis in industrial crop Jerusalem artichoke (Helianthus tuberosus L.) leaves. Ind. Crops Prod. 47, 339–345 (2013)
Boyraz, N.; Özcan, M.: Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. Int. J. Food Microbiol. 107, 238–242 (2006)
Han, K.S.; Lee, S.C.; Lee, J.S.; Soh, J.W.: First report of pink mold rot on tomato fruit caused by Trichothecium roseum in Korea. Res. Plant Dis. 18, 396–398 (2012). doi:10.5423/RPD.2012.18.4.396
Shamsi, S.; Sultana, R.: Trichothecium roseum link—a new record of hyphomycetous fungus for Bangladesh. Bangladesh J. Plant Taxon. 15, 77–80 (2008)
Zabka, M.; Drastichova, K.; Jegorov, A.; Soukupova, J.; Nedbal, L.: Direct evidence of plant-pathogenic activity of fungal metabolites of Trichothecium roseum on apple. Mycopathologia 162, 65–68 (2006)
Oh, S.Y.; Nam, K.W.; Yoon, D.H.: Identification of Acremonium acutatum and Trichothecium roseum isolated from grape with white stain symptom in Korea. Mycobiology 42, 269–273 (2014)
Shukla, A.C.: Plant secondary metabolites as source of postharvest disease management; an overview. J. Stored Prod. Postharvest Res. 4, 1–10 (2013)
Al-Reza, S.M.; Rahman, A.; Ahmed, Y.; Kang, S.C.: Inhibition of plant pathogens in vitro and in vivo with essential oil and organic extract of Cestrum nocturnum L. Pestic. Biochem. Physiol. 96, 86–92 (2010)
Soylu, E.M.; Kurt, Ş.; Soylu, S.: In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 143, 183–189 (2010)
Passone, M.A.; Girardi, N.S.; Etcheverry, M.: Antifungal and antiaflatoxigenic activity by vapor contact of three essential oils, and effects of environmental factors on their efficacy. LWT Food Sci. Technol. 53, 434–444 (2013)
Baba, S.A.; Malik, S.A.: Evaluation of antioxidant and antibacterial activity of methanolic extracts of Gentiana kurroo royle. Saudi J. Biol. Sci. 21(5), 8–493 (2014)
De Souza, E.L.; De Oliveira Lima, E.; De Luna Freire, K.R.; De Souza, C.P.: Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Braz. Arch. Biol. Technol. 48, 245–250 (2005)
Tegegne, G.; Pretorius, J.; Swart, W.: Antifungal properties of Agapanthus africanus L. extracts against plant pathogens. Crop Prot. 27, 1052–1060 (2008)
Luz, C.; Netto, M.C.; Rocha, L.F.: In vitro susceptibility to fungicides by invertebrate-pathogenic and saprobic fungi. Mycopathologia 164, 39–47 (2007)
Abdel-Monaim, M.F.; Abo-Elyousr, K.A.M.; Morsy, K.M.: Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinus termis Forsik). Crop Prot. 30, 185–191 (2011)
Askarne, L.; Talibi, I.; Boubaker, H.; Amine, S.M.; Boudyach, E.H.; Ait Ben Aoumar, A.: Effects of organic acids and salts on the development of Penicillium italium: the causal agent of citrus blue mould. Plant Pathol. J. 10, 99–107 (2011)
Askarne, L.; Talibi, I.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Serghini, M.A.; Ait Ben Aoumar, A.: In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mould. Crop Prot. 40, 53–58 (2012)
CLSI: Clinical and Laboratory Standarts Institute, formerly NCCLS, National Committee for Clinical and Laboratory Standarts. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved Standard, 2nd edn. NCCLS document M27-A2, NCCLS, Wayne, PA. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved Standard, 1st edn. NCCLS document M 38 A, Wayne, PA (2002)
Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A.: Antifungal activity of some Moroccan plants against Geotrichum candidum, the causel agent of postharvest citrus sour rot. Crop Prot. 35, 41–46 (2012)
Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.: Analysis of total phenol sandother oxidation substrates and antioxidants by means of folin-ciocalteure agent. Methods Enzymol. 299, 152–178 (1999). doi:10.1016/S0076-6879(99)99017-1
Arnous, A.; Makris, D.P.; Kefalas, P.: Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 49, 5736–5742 (2001)
Baydar, N.G.; Baydar, H.: Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extract. Ind. Crops Prod. 41, 375–380 (2013)
Caponio, F.; Alloggio, V.; Gomes, T.: Phenolic compounds of virgin olive oil: influence of paste preperation techniques. Food Chem. 64, 203–209 (1999)
Baba, S.A.; Malik, S.A.: Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 9(4), 449–454 (2015)
Baba, S.A.; Malik, A.H.; Wani, Z.A.; Mohiuddin, T.; Shah, Z.; Abbas, N.; Ashraf, N.: Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S. Afr. J. Bot. 99, 80–87 (2015)
Bolis, M.S.: Antioxidants determination by the use of a stable free radical. Nature 181, 1199–1200 (1958)
Parvu, M.; Vlase, L.; Fodorpataki, L.; Parvu, O.; Roscacasian, O.; Bartha, C.; Barbu-Tudoran, L.; Parvu, A.E.: Chemical composition of Celandine (Chelidonium majus L.) extract and its effects on Botrytis tulipae (Lib.) lind fungus and the Tulip. Not. Bot. Horti. Agrobot. 41(2), 414–426 (2013)
Stefanović, O.; Radojević, I.; Vasić S.; Čomić, L.: Antibacterial activity of naturally occurring compounds from selected plants, In: Antimicrobial Agents. In Tech, pp.1–24 (2012)
Mahboubi, A.; Kamalinejad, M.; Shalviri, M.; Karbasi, Z.; Jafariazar, Z.; Asgharian, R.: Evaluation of antibacterial activity of three Iranian medicinal plants. Afr. J. Microbiol. Res. 6, 2048–2052 (2012)
Knežević, S.V.; Ivan Kosalec, I.; Babac, M.; Petrović, M.; Ralić, J.; Matica, B.; Blažeković, B.: Antimicrobial activity of Thymus longicaulis C. Presl essential oil against respiratory pathogens. Cent. Eur. J. Biol. 7, 1109–1115 (2012)
Kocić-Tanackov, S.D.; Dimić, G.R.; Tanackov, I.J.; Pejin, D.J.; Mojović, L.V.; Pejin, J.D.: Antifungal activity of Oregano (Origanum vulgare L.) extract on the growth of Fusarium and Penicillium species isolated from food. Hem. Ind. 66, 33–41 (2012)
Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H.: Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 15, 549–557 (2004)
Deressa, T.; Wakjira, M.: Antifungal activity of some invasive alien plant leaf extracts against mango (Mangiferaindica) anthracnose caused by Colletotrichum gloeosporioides. Int. J. Pest Manag. 61, 99–105 (2015)
Carvalho, D.D.C.; Alves, E.; Camargos, R.B.; Oliveira, D.F.; Scolforo, J.R.S.; De Carvalho, D.A.; Batista, T.R.S.: Plant extracts to control alternaria alternate in Murcott tangor fruits. Rev. Iberoam. Micol. 28, 173–178 (2011)
Garduno-Pizana, C.; Barrera-Necha, L.L.; Gomez, Y.R.: Evaluation of the fungicidal activity of leaves powders and extracts of fifteen Mexican plants against Fusarium oxysporum f. sp. gladioli. Plant Pathol. J. 9, 103–111 (2010)
Amsalu, A.; Fikire, L.; Diriba, M.: The antifungal activity of some medicinal plants against coffee berry disease caused by Colletotrichum kahawe. Int. J. Agric. Res. 6, 268–279 (2011)
Wang, J.; Li, J.; Cao, J.; Jiang, W.: Antifungal activities of neem (Azadirachta indica) seed kernel extracts on postharvest diseases in fruits. Afr. J. Microbiol. Res. 4, 1100–1104 (2010)
Cutter, C.N.: Antimicrobial effect of herb extracts against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella typhimurium associated with beef. J. Food Prot. 63, 601–607 (2000)
Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V.: Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 6, 1451–1474 (2013). doi:10.3390/ph6121451
Jakovljević, Z.D.; Stanković, M.S.; Topuzović, D.M.: Seasonal variability of Chelidonium majus L. secondary metabolites content and antioxidant activity. EXCLI J. 12, 260–268 (2013)
Ebrahimzade, M.A.; Nabavi, S.F.; Nabav, S.M.: Antioxidant activities of methanol extract of Sambucus ebulus L. Flower. Pak. J. Biol. Sci. 12, 447–450 (2009)
Sarikurkcu, C.; Sabih Ozer, M.; Eskici, M.; Tepe, B.; Can, S.; Mete, E.: Essential oil composition and antioxidant activity of Thymus longicaulis C. Presl subsp. longicaulis var. Longicaulis. Food Chem. Toxicol. 48, 1801–1805 (2010)
Trigui, M.; Hsouna, A.B.; Tounsi, S.; Jaoua, S.: Chemical composition and evaluation of antioxidant and antimicrobial activities of Tunisian Thymelaea hirsute with special reference to its mode of action. Ind. Crops Prod. 41, 150–157 (2013)
Duke, S.O.; Baerson, S.R.; Dayan, F.E.; Rimando, A.M.; Scheffler, B.E.; Tellez, M.R.; Wedge, D.E.; Schrader, K.K.; Akey, D.H.; Arthur, F.H.; Lucca, A.J.D.; Gibson, D.M.; Harrison, H.F.; Peterson, J.K.; Gealy, D.R.; Tworkoski, T.; Wilson, C.L.; Morris, J.B.: United States Department of Agriculture—Agricultural Research Service research on natural products for pest management. Pest. Manag. Sci. 59, 708–717 (2003)
Wen, A.; Delaquis, P.; Stanich, K.; Toivonen, P.: Antilisterial activity of selected phenolic acids. Food Microbiol. 20, 305–311 (2003)
Sisti, M.; De Santi, M.; Fraternale, D.; Ninfali, P.; Scoccianti, V.; Brandi, G.: Antifungal activity of Rubus ulmifolius Schott standardized in vitro culture. LWT Food Sci. Technol. 41, 946–950 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balkan, B., Balkan, S., Aydoğdu, H. et al. Evaluation of Antioxidant Activities and Antifungal Activity of Different Plants Species Against Pink Mold Rot-Causing Trichothecium roseum . Arab J Sci Eng 42, 2279–2289 (2017). https://doi.org/10.1007/s13369-017-2484-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-017-2484-4