Abstract
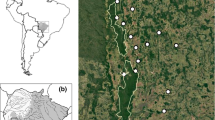
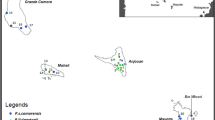
It is commonly assumed that bats, due to their flight capacity, are not affected by landscape attributes across small geographic extensions. However, recent studies with phyllostomids have found evidence of negative responses, such as decreasing genetic diversity with decreasing forest amount, specifically in areas dominated by agricultural land. The purpose of this study was to evaluate if landscape composition and configuration could be influencing the genetic diversity of a common frugivorous bat: Artibeus jamaicensis. We worked in an area characterized by the presence of extensive agricultural land, with a trend towards open spaces of high contrast with forests. Through mtDNA control region sequences, we inferred high levels of genetic diversity in the surveyed landscapes. In order to determine a possible relationship between genetic diversity and landscape attributes, we employed a multivariate exploratory analysis that allowed us to determine the independent contribution of each variable, in a hierarchical model. We found a negative relationship between genetic diversity and total forest edge, which is a variable that reflects the degree of fragmentation. This procedure can be implemented in population genetics, allowing the incorporation of spatially explicit variables.


Similar content being viewed by others
References
American Museum of Natural History [AMNH] (2018) Wing Punch and Hair Sampling Protocols. Available from: http://research.amnh.org/vz/mammalogy/donating-bat-tissue-and-hair-samples-genomic-and-stable-isotope-studies/wing-punch-and-hair-sampling
Anderson CD, Epperson BK, Fortin MJ, Holderegger R, James PMA, Rosenberg MS, Scribner KT, Spear S (2010) Considering spatial and temporal scale in landscape-genetic studies of gene flow. Mol Ecol 19:3565–3575
Anthony ELP (1988) Age determination in bats: In: Kunz TH (ed). Ecological and behavioral methods for the study of bats. Smithsonian Institution Press, Washington, DC, pp 47–58
Arroyo-Rodríguez V, Rojas C, Saldaña-Vásquez RA, Stoner KE (2016) Landscape composition is more important than landscape configuration for phyllostomid bat assemblages in a fragmented biodiversity hotspot. Biol Conserv 198:84–92
Ávila-Cabadilla LD, Sánchez-Azofeifa GA, Stoner KE, Álvarez-Añorve MY, Quesada M, Portillo-Quintero CA (2012) Local and landscape factors determining occurrence of Phyllostomid bats in tropical secondary forests. PLoS One 7(4):e35228
Baguette M, Blanchet S, Legrand D, Stevens VM, Turlure C (2013) Individual dispersal, landscape connectivity and ecological networks. Biol Rev 88:310–326
Balkenhol N, Cushman SA, Storfer A, Waits LP (2016) Introduction to landscape genetics-concepts, methods, applications. In: Balkenhol N, Chushman SA, Storfer AT, Waits LP (eds) Landscape genetics: concepts, methods, applications. Wiley Blackwell, UK, pp 1–7
Bennett AF, Saunders DA (2010) Habitat fragmentation and landscape change. In: Sodhi NS, Ehrlich PR (eds) Conservation biology for all. Oxford University Press, New York, pp 88–104
Bernard E, Fenton MB (2003) Bat mobility and roosts in a fragmented landscape in Central Amazonia, Brazil. Biotropica 35(2):262–277
Bolívar-Cimé B, Laborde J, MacSwiney MC, Muñoz-Robles C, Tun-Garrido J (2013) Response of phytophagous bats to patch quality and landscape attributes in fragmented tropical semi-deciduous forest. Acta Chiropterol 15(2):399–409
Bolton PE, West AJ, Cardilini APA, Clark JA, Maute KL, Legge S, Brazill-Boast J, Griffith SC, Rollins LA (2016) Three molecular markers show no evidence of population genetic structure in the Gouldian finch (Erythrura gouldiae). PLoS One 11(12):e0167723
Burland TM, Worthington-Wilmer J (2001) Seeing in the dark: molecular approaches to the study of bat populations. Biol Rev 76:389–409
Carstens BC, Sullivan J, Davalos LM, Larsern PA, Pedersen SC (2004) Exploring population genetic structure in three species of Lesser Antillean bats. Mol Ecol 13:2557–2566
Chevan A, Sutherland M (1991) Hierarchical partitioning. Am Stat 45(2):90–96
Comisión Nacional de Áreas Naturales Protegidas [CONANP] (2007) Programa de conservación y manejo del Parque Nacional Lagunas de Montebello. SEMARNAT, Mexico City
Corthals A, Martin A, Warsi OM, Woller-Skar M, Lancaster W, Russell A, Dávalos LA (2015) From the field to the lab: best practices for field preservation of bat specimens for molecular analysis. PLoS One 10(3):e0118994
Cushman SA, McGarigal K, Neel MC (2008) Parsimony in landscape metrics: strength, universality, and consistency. Ecol Indic 8:691–703
Davy CM, Martínez-Núñez F, Willis CKR, Good SV (2015) Spatial genetic structure among bat hibernacula along the leading edge of a rapidly spreading pathogen. Conserv Genet 16(5):1013–1024
De la Peña-Cuéllar E, Benítez-Malvido J, Ávila-Cabadilla LD, Martínez-Ramos M, Estrada A (2014) Structure and diversity of phyllostomid bats assemblages on riparian corridors in a human-dominated tropical landscape. Ecol Evol 5(4):903–913
Diniz-Filho JAF, Soares TN, Lima JS, Dobrovolski R, Lemes-Landeiro V, Telles MP, Rangel TF, Bini LM (2013) Mantel test in population genetics. Genetics Mol Biol 36(4):475–485
Diniz-Filho JAF, Telles MP, Bonatto SL, Eizirik E, de Freitas TRO, de Marco P, Santos FR, Sole-Cava A, Nascimiento-Soares T (2008) Mapping the evolutionary twilight zone: molecular markers, populations and geography. J Biogeogr 35:753–763
Dixo M, Metzger JP, Morgante JS, Zamudio KR (2009) Habitat fragmentation reduces diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biol Conserv 142:1560–1569
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of population. Mol Ecol 11:2571–2581
Eastman JR (2012) IDRISI selva tutorial: manual versión 17. Clark Labs, Clark University
Ethier K, Fahrig L (2011) Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landsc Ecol 26:865–876
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinforma 1:47–50
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Fahrig L (1999) Forest lost and fragmentation: which has the greater effect on persistence of forest-dwelling animals. In: Rochelle JA, Lehman LA, Wisniewski J (eds) Forest fragmentation: wildlife and management implication. Brill Press, Netherlands, pp 87–95
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fleming TH, Murray KL (2009) Population and genetic consequences of hurricanes for three species of West Indian phyllostomid bats. Biotropica 42(2):250–256
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Frankham R, Ballou JD, Briscoe DA (2002) Genetic diversity. Introduction to conservation genetics. Cambridge University Press, New York, pp 45–71
García-García JL, Santos-Moreno A (2014) Efectos de la estructura del paisaje y de la vegetación en la diversidad de murciélagos filostómidos (Chiroptera: Phyllostomidae) de Oaxaca, México. Rev Biol Trop 62(1):217–239
Gonçalves da Silva A, Gaona O, Medellín RA (2008) Diet and trophic structure in a community of fruit-eating bats in Lacandon forest, Mexico. J Mammal 89(1):43–49
Handley CO, Gardner AL, Wilson DE (1991) Movements. In: Handley CO, Wilson DE, Gardner AL (eds) Demography and natural history of the common fruit bat, Artibeus jamaicensis, on Barro Colorado Island, Panama. Smithsonian Institution Press, Washington, DC, pp 89–130
Heim O, Treitler JT, Tschapka M, Knörnschild M, Jung K (2015) The importance of landscape elements for bat activity and species richness in agricultural areas. PLoS One 10:e0134443
Höglund J (2009) Evolutionary conservation genetics. Oxford University Press, USA, pp 60–80
Holderegger R, Wagner HH (2008) Landscape genetics. Bioscience 58:199–207
Jackson ND, Fahrig L (2016) Habitat amount, not habitat configuration, best predicts population genetic structure in fragmented landscapes. Landsc Ecol 31:951–968
Kraker-Castañeda C, Santos-Moreno A, Lorenzo C, Horváth A, MacSwiney MC, Navarrete-Gutiérrez D (2017) Responses of phyllostomid bats to forest cover in upland landscapes in Chiapas, southeast Mexico. Stud Neotropical Fauna Environ 52(2):112–121
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R et al (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 Available from: http://www.clustal.org/clustal2/
Larsen PA, Hoofer SR, Bozeman MC, Pedersen SC, Genoways HH, Carleton JP, Pumo DE, Baker RJ (2007) Phylogenetics and phylogeography of the Artibeus jamaicensis complex based on cytochrome-b DNA sequences. J Mammal 88(3):712–727
Laurance WF (2014) Contemporary drivers of habitat fragmentation. In: Kettle CJ, Pin Koh L (eds). CAB International, p 20–27
Librado P, Rozas J (2009) DnaSP v.5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452 Available from: www.ub.edu./dnasp/index_v5.html
Lindenmayer DB, Fischer J (2006) Habitat fragmentation and landscape change: an ecological and conservation synthesis. Island Press, Washington DC, pp 15–25
Llaven-Macías V, Ruiz-Montoya L, García-Bautista M, Lesher-Gordillo J, Machkour M’Rabet S (2017) Genetic diversity and structure of Artibeus jamaicensis (Chiroptera: Phyllostomidae) in Chiapas, Mexico. Acta Zool Mex 33(1):55–66
Lu D, Weng Q (2007) A survey of image classification methods and techniques for improving classification performance. Int J Remote Sens 28(5):823–870
Mac Nally R (2000) Regression and model-building in conservation biology, biogeography and ecology: the distinction between -and reconciliation of- ‘predictive’ and ‘explanatory’ models. Biodivers Conserv 9:655–671
Mac Nally R (2002) Multiple regression and inference in ecology and conservation biology: further comments on identifying important predictor variables. Biodivers Conserv 11:1397–1401
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trend Ecol Evol 18(4):189–197
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27(2):209–220
Martínez-Sánchez J (2005) Frugívoros voladores y la dispersión de semillas en el Parque Nacional Lagunas de Montebello, Chiapas, México. (Master’s thesis). El Colegio de la Frontera Sur (ECOSUR)
McCulloch ES, Tello SJ, Whitehead A, Rolón-Mendoza CMJ, Maldonado-Rodríguez MCD, Stevens RD (2013) Fragmentation of Atlantic Forest has not affected gene flow of a widespread seed-dispersing bat. Mol Ecol 22:4619–4633
McGarigal K (2015) Fragstats help. Fragstats 4.2: Spatial pattern analysis program for categorical and continuous maps. University of Massachusetts
McGarigal K, Ene E (2013) Fragstats 4.2 v4.2.1.603: spatial pattern analysis program for categorical and continuous maps. University of Massachusetts, Amherst Available from: http://www.umass.edu/landeco/research/fragstats/fragstats.html
McGarigal K, Marks BJ (1995) Fragstats. Spatial pattern analysis program for quantifying landscape structure. Version 2.0. USDA Forest Service General Technical Report
Meyer CFJ, Kalko EKV, Kerth G (2009) Small-scale fragmentation effects on local genetic diversity in two phyllostomid bats with different dispersal abilities in Panama. Biotropica 41(1):95–102
Meyer CFJ, Struebig MJ, Willig MR (2016) Responses of tropical bats to habitat fragmentation, logging, and deforestation. In: Voigt CC, Kingston T (eds) Bats in the Anthropocene: conservation of bats in a changing world. Springer International Publishing, Switzerland, pp 63–103
Morrison DW (1978a) Foraging ecology and energetics of the frugivorous bat Artibeus jamaicensis. Ecology 59(4):716–723
Morrison DW (1978b) Influence of habitat on the foraging distances of the fruit bat, Artibeus jamaicensis. J Mammal 59(3):622–624
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nei M, Li W (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases (molecular evolution/mitochondrial DNA/nucleotide diversity). Proc Natl Acad Sci U S A 76(10):5269–5273
Norberg UM, Rayner MV (1987) Ecological morphology and flight in bats (Mammalia: Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc Lond Ser B Biol Sci 326(1179):335–427
Olea PP, Mateo-Tomás P, de Frutos A (2010) Estimating and modelling bias of the hierarchical partitioning public-domain software: implications in environmental management and conservation. PLoS One 5(7):e11698
Ortega J, Castro-Arellano I (2001) Artibeus jamaicensis. Mamm Species 662:1–9
Ortega J, Maldonado JE, Wilkinson GS, Arita HT, Fleischer RC (2003) Male dominance, paternity, and relatedness in the Jamaican fruit-eating bat (Artibeus jamaicensis). Mol Ecol 12:2409–2415
Pinto N, Keitt TH (2008) Scale-dependent responses to forest cover displayed by frugivore bats. Oikos 117:1725–1731
Pulido-Solís MT (2000) Haciendas de Chiapas. CONECULTA-Chiapas, Tuxtla Gutiérrez
Pumo DE, Goldin EZ, Elliot B (1988) Mitochondrial DNA polymorphism in three Antillean island populations of the fruit bat, Artibeus jamaicensis. Mol Biol 5(1):79–89
Pumo DE, Kim I, Remsen J, Phillips CJ, Genoways HH (1996) Molecular systematics of the fruit bat, Artibeus jamaicensis: origin of an unusual island population. J Mammal 77(2):491–503
Ramírez-Marcial N, González-Espinosa M, Camacho-Cruz A, Ortiz-Aguilar D (2010) Forest restoration in Lagunas de Montebello National Park, Chiapas, Mexico. Ecol Restor 28(3):354–360
Ripperger SP, Tschapka M, Kalko EKV, Rodriguez-Herrera B, Mayer F (2013) Life in a mosaic landscape: anthropogenic habitat fragmentation affects genetic population structure in a frugivorous bat species. Conserv Genet 14:925–934
Ripperger SP, Tschapka M, Kalko EKV, Rodriguez-Herrera B, Mayer F (2014) Resisting habitat fragmentation: high genetic connectivity among populations of the frugivorous bat Carollia castanea in an agricultural landscape. Agric Ecosyst Environ 185:9–15
Ruiz EA, Vargas-Miranda B, Zúñiga G (2013) Late-Pleistocene phylogeography and demographic history of two evolutionary linages of Artibeus jamaicensis (Chiroptera: Phyllostomidae) in Mexico. Acta Chiropterol 15(1):19–33
Ruz MH (1992) Savia india, floración ladina. Apuntes para una historia de las fincas comitecas (siglos XVIII y XIX). CONACULTA, Mexico city
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5(1):18–32
Sikes RS, the Animal Care and Use Committee of the American Society of Mammalogists (2016) 2016 guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 92(1):235–253
Tajima F (1983) Evolutionary relationship of DNA sequences in finite populations. Genetics 105:437–460
Turner MG, Gardner RH, RV O’N (2001) Landscape ecology in theory and practice: pattern and process. Springer-Verlag, New York
Vázquez-Domínguez E, Mendoza-Martínez A, Orozco-Lugo L, Cuarón AD (2013) High dispersal and generalist habits of the bat Artibeus jamaicensis on Cozumel Island, México: an assessment using molecular genetics. Acta Chiropterol 15(2):411–421
Verbyla DL (1995) Satellite remote sensing of natural resources. CRC Press, Boca Raton
Walsh C, Mac Nally R (2013) hier.part: hierarchical partitioning. R Package, version 1.0–4. Available from: http://cran.au.r-project.org
Wan QH, Wu H, Fujihara T, Fang SG (2004) Which genetic marker for which conservation genetics issue? Electrophoresis 25:2165–2176
Wang X, Blanchet FG, Koper N (2014) Measuring habitat fragmentation: an evaluation of landscape patter metrics. Methods Ecol Evol 5(7):634–646
Worthington-Wilmer JW, Barratt E (1996) A non-lethal method of tissue sampling for genetic studies of chiropteran. Bat Res News 37:1–3
Wright S (1943) Isolation by distance. Genetics 28(2):114–138
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7(1–2):203–214
Acknowledgements
We thank C. Lorenzo and J. Bolaños from the Colección Mastozoológica (ECOSUR-SCLC), for housing the tissue samples. We appreciate the support of the PNLM-CONANP authorities, staff and park rangers, especially O. Cervantes and A. León, as well as the support of G. Lalo Jacinto and the authorities of the Instituto Nacional de Antropología e Historia (INAH), for permission to work in the Chinkultic archeological site. We are grateful with G. Castellanos, B. Cruz and C. Lorenzo for the revision of early versions of this manuscript. The Instituto Politécnico Nacional (IPN) of Mexico provided support during fieldwork. Finally, we thank landowners and local authorities for permission to work in the area.
Funding
This project was partially funded by Idea Wild. E. M. Leiva-González received a scholarship (No. 597881) provided by the Consejo Nacional de Ciencia y Tecnología (CONACyT-Mexico).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Joanna Stojak
Rights and permissions
About this article
Cite this article
Leiva-González, E.M., Navarrete-Gutiérrez, D., Ruiz-Montoya, L. et al. Analysis of the contribution of landscape attributes on the genetic diversity of Artibeus jamaicensis Leach, 1821. Mamm Res 64, 223–233 (2019). https://doi.org/10.1007/s13364-018-0403-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-018-0403-z