Abstract
Although the paca is the most frequently hunted game species throughout the Neotropics, its behavioral and ecological requirements remain poorly understood. Here, we describe ranging behavior, spatio-temporal intraspecific interactions, and cavity use within a mosaic landscape in Central Belize, based on radio-tracking of four males and two females. This study is the first to investigate social interactions and spatial structure within a paca population in unprotected marginal habitat. We detected extensive home range overlap between males and between sexes. Male-male overlap was less extensive within core areas, while female core ranges were almost entirely occupied by one or more males. On average, pacas used at least six cavities within their home range. The majority of cavities were in the core areas and we did not detect simultaneous co-habitation of the same location. On average, females occupied cavities that were closer together, and closer to water bodies, than those of males. Overall, our study suggests a general tolerance during nocturnal foraging activities, but exclusive use of core areas and associated cavities. The larger ranges of males than females and the extensive overlap between conspecifics suggest a polygamous or promiscuous mating system in this landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compared to tropical environments in Asia and Africa, the Neotropics are characterized by few large ground-dwelling vertebrate species, providing a limited range of heavily exploited game species (Fa and Peres 2001; Fritz and Loison 2006). Overhunting of terrestrial vertebrates threatens food security livelihoods, alters patterns of forest regeneration, and can have cascading effects on other taxa (Stoner et al. 2007; Gordon et al. 2012; Reider et al. 2013). Preventing “empty forests” and the plethora of associated detrimental consequences (Redford 1999), and maintaining viable populations and ecosystem function will require careful management of game harvests. Sustainable management of harvested wild populations requires understanding their spatial distributions and social structures, as both may influence recovery from local reductions in abundance (e.g., Stoner et al. 2006; Ausband et al. 2015). Spatial, demographic, and social parameters are well understood for popular game and trophy species in areas that have a long history of studying and regulating harvests (e.g., Europe, North America, and sub-Saharan Africa: Coltman et al. 2003; Milner et al. 2007; Proffitt et al. 2010; Ordiz et al. 2012; Mysterud 2014; Lone et al. 2015). Such detailed knowledge is lacking from the Neotropics, despite its long tradition of subsistence and commercial hunting, both for wild meat and body parts (Pérez 1992; Ojasti 1996).
One of the most common and frequently hunted game species throughout the Neotropics is the paca (Cuniculus paca), a large, nocturnal caviomorph rodent; currently of least concern on the IUCN Red List, but suffering local extinctions in the southern part of its range (Pérez 1992; Escamilla et al. 2000; Altrichter 2001; Smith 2005; Koster 2008; Emmons et al. 2016; Foster et al. 2016). Despite its widespread exploitation and economic value, the paca’s spatial and behavioral ecology is poorly understood. Few studies have quantified home range sizes of pacas, but those that have suggest that male and female ranges vary with habitat quality and the productivity and patchiness of food resources, with the largest ranges documented in a mosaic of marginal habitats, and the smallest in primary broadleaf forest (Marcus 1984; Beck-King et al. 1999; Ulloa et al. 1999; Gutierrez et al. 2016). While Marcus (1984) found no difference in range size between the sexes, Gutierrez et al. (2016) observed male ranges up to four times the size of female ranges.
Pacas are considered solitary, territorial, and aggressively intolerant of conspecifics, with a monogamous or perhaps promiscuous mating system, and parental care provided by the female only (Marcus 1984; Smythe and Brown de Guanti 1995; Herrera 2016). Pacas forage opportunistically at night, primarily on the pulp of fruits that have fallen to the forest floor, and, to a lesser extent, on seeds (Marcus 1984; Pérez 1992; Laska et al. 2003; Dubost and Henry 2006). Although adults generally forage alone or with their young (Collet 1981; Marcus 1984), groups of up to five individuals have been observed foraging in areas with a high abundance of food (Beck-King et al. 1999) suggesting a degree of social tolerance under certain conditions. However, Marcus (1984) observed closely overlapping ranges between mated pairs, whose ranges did not overlap with adjacent pairs, suggesting intolerance with monogamous exclusive ranges.
During the day, pacas occupy cavities such as hollow logs and spaces below root formations, usually alone or with young (Marcus 1984; Aquino et al. 2012; Figueroa-de-León et al. 2016). The cavities are often highly complex, with chambers, connecting tunnels, and multiple openings for escape from predators (Aquino et al. 2009; Aquino et al. 2012). Paca cavities are often associated with water, as they defecate in water to avoid leaving spoor which may attract predators, and if threatened, they escape predators by leaping into water, submerging, and swimming (Pérez 1992; Smythe and Brown de Guanti 1995; Aquino et al. 2009; Figueroa-de-León et al. 2016).
The emerging picture is of a spatial distribution and social system that varies with the availability of limited resources, with females distributed according to food, suitable den sites, and predator-free space (access to water), and males distributed according to females. Under conditions of abundant food, females may have reduced home ranges and be more tolerant of competitors while foraging; the defense of suitable cavity sites or access to water may be more important when rearing young. Understanding how the spatial distribution and social structure of pacas varies with the availability of resources such as food and suitable resting sites will aid the management of paca populations on a regional scale across a range of landscapes.
Our study is the first investigation of spatio-temporal interactions amongst pacas in marginal habitat. We describe ranging behavior, intraspecific interactions, and cavity use by pacas within a mosaic landscape of settlements, pine savannah, and secondary broadleaf forest in Central Belize over a period of 2 years. We use telemetry data for six pacas, which we previously used to estimate paca home range size and habitat selection, documenting the largest detected home ranges for this species to date, but without investigating overlap or shared use of resources (Gutierrez et al. 2016). Here, we address that hiatus by investigating interactions between the pacas through space and time. Our study area is considered marginal for pacas, with competition for limited resources as well as seasonal flooding and poor drainage (Gutierrez et al. 2016), potentially limiting the availability of suitable refuges for daytime resting and access to water bodies in the dry season. We therefore expected exclusive home ranges associated with intolerance towards conspecifics of the same sex and the defense of resting sites.
Materials and methods
Study area
Our study focused on a ~ 10-km2 area within the Central Belize Corridor (“CBC”, location 17° 21′ 37.0″ N, 88° 33′ 37.6″ W; Doncaster et al. 2012; Kay et al. 2015). The CBC is a 750-km2 mosaic landscape of lowland secondary broad-leaved moist forest, short-grass savanna, shrubland, wetland, and agriculture which links the Selva Maya forest of Northern Belize, Guatemala, and Mexico and the Maya Moutain Massif forest of Southern Belize. The landscape supports a low human density of approximately 5 people/km2 across seven settlements and is bisected by a two-lane highway. Human activities include livestock farming, citrus and sugarcane plantations, slash-and-burn farms, game hunting, and logging. Average annual rainfall from 2010 to 2012 was 180 mm, with most falling within the wet season (June to November) and average temperatures ranged from 21.6 to 31.5 °C (Gutierrez et al. 2016). The area is low-lying (30-45 m above sea level). The 10-km2 study area included five permanent natural and man-made water holes and a seasonal creek.
Trapping, radio telemetry, and home range estimation
Pacas were trapped, collared, and tracked between May 2010 and February 2012. Live traps (Tomahawk, Hazelhurst, WI, USA) were deployed on game trails and areas with signs of paca activity and baited with fruit. Trapped pacas were immobilized using ketamine hydrochloride (48.9 mg/kg) and acepromazine maleate (0.6 mg/kg) and fitted with motion sensitive VHF radio collars (MOD-125, Telonics Inc., Meza AZ, USA). All animal handling was in accordance with permits (CD/60/3/09(18) and CD/60/3/10(44)) issued by the government of Belize. Pacas were recaptured when the radio collars were nearing the end of their battery life, upon which collars were removed and the animals released after anesthetic reversal. Collared pacas were located via triangulation, keeping inter-bearing angles between ≥ 20° and ≤ 170°. To increase accuracy, paca locations were taken simultaneously by three or more observers from different locations (via radio contact). Locations of pacas were recorded at 15-min intervals for a maximum of 5 h. The majority of locations were taken between dusk and dawn when pacas are most active (Harmsen et al. 2010). Pacas tend to remain in cavities during daylight hours, allowing observers to find their precise locations when taking daytime azimuths.
Two adult females and four adult males were tracked between June 2010 and February 2012. Of these, a male and female paca were tracked simultaneously for 2 months; thereafter, four pacas were tracked simultaneously for periods ranging from 2 to 7 months, dependent on the capture dates. Home range sizes by minimum convex polygon (MCP) and core areas by kernel (KHR) are reported in Gutierrez et al. (2016). We additionally performed a bootstrapping procedure to assess whether enough locations were gained for each paca individual to reliably estimate home range sizes, using the package move in R (Kranstauber and Smolla 2016). We achieved this by accumulatively adding locations, which were randomly chosen from the total dataset per individual, in a stepwise logarithmic fashion. The procedure was repeated 100 times at each accumulative step, allowing us to calculate the mean and variance of increased MCP size with accumulative points and to visualize to what extent an asymptote is reached when 95% of the points are included.
Static interactions
Static interaction analysis informs on spatial attraction or avoidance by quantifying the level of overlap between two spatial utility distributions (UDs), independent of time. Static interaction indices consist of a single number representing the level of overlap. By taking into account the UD of individuals, these indices provide a more accurate representation of space sharing between pairs than calculating the percentage of area overlap (Fieberg and Kochanny 2005). We calculated home range overlap (HRO) between pairs of individuals using the adehabitatHR package (Calenge 2006) in R, calculating the utilization distribution overlap index (UDOI), similar to Hulbert’s index of niche overlap (Fieberg and Kochanny 2005). The UDOI ranges from 0 (no overlap) to 1 (complete overlap of uniform home ranges) and may exceed 1 if the UD is non-uniform with a high degree of overlap between individuals.
We calculated overlap of home-range kernels (95% KHR) and core areas (50% KHR) between pairs of simultaneously tracked pacas using UDOI. We compared 95% KHR UDOI (range overlap) with the 50% KHR UDOI (core area overlap) using a paired Student’s t test.
We compared the HROs of male-male and male-female pairs using Student’s t tests. We could not test for interaction between the females because they were not tracked simultaneously.
For four pacas with sufficient data points, we additionally tested for temporal shifts in each home range by partitioning the locations for an individual into two time periods and calculating the 95% KHR UDOI.
Dynamic interactions
Home range overlap does not take into account the temporal aspect of animal interactions. We used dynamic interaction (DI) indices to test for concurrent associations of attraction, avoidance, or indifference between pairs of individuals with locations taken in the same 15-min time blocks. Indices of DI are susceptible to type I error (Long et al. 2014). We therefore estimated and compared three point-based indices of DI to assess the robustness of the results: (1) Doncaster’s (1990) non-parametric test of interaction, testing for significantly high or low probabilities of cumulative separations between two animals; (2) Kenward et al.’s (1993) coefficient of sociality, Cs, which ranges from − 1 (avoidance) to 1 (attraction); and (3) Benhamou et al.’s (2014) interaction statistic (IAB), which ranges from 0 (avoidance) to 1 (attraction). We assumed a critical distance threshold of 100 m, within which pacas would be aware of each other due to their enhanced visual, auditory, and olfactory senses (Macdonald 2013), and following other studies examining DI (e.g., Quaglietta et al. 2014). We used the package adehabitatLT (Calenge 2006) for computation of Doncaster’s (1990) and Kenward et al.’s (1993) indices and wildlifeDI tools (Long et al. 2014) for IAB; in R. Additionally, we calculated the distances between simultaneous location fixes of pacas using wildlifeDI tools (Long et al. 2014) to explore close proximity encounters (e.g., mating or antagonistic events).
Cavity use
As pacas are sedentary during the day, we were not constrained by the risk of disrupting their movement when taking daytime location fixes. Therefore, compared to night fixes, day fixes could be taken from more angles, increasing the accuracy of our estimate of the resting locations.
We classified day fixes as those occurring between 07:30 and 17:00 and assumed that they represented the daytime resting cavity, as this falls outside pacas’ main activity period (Beck-King et al. 1999; Harmsen et al. 2010). Resting locations were not monitored daily; however, we counted the minimum number used by each paca within the monitored period, calculated the percentage that occurred within individuals’ core areas, and tested for differences between sexes using Student’s t test. In order to understand whether pacas use cavities opportunistically (i.e., occupying the nearest suitable cavity after a night of foraging), or preferentially return to specific cavities, we applied a Student’s t test to the difference between the average distance of the day location from the last known night location, and the average distance of the day location from all of the previous night’s locations. We hypothesized that if pacas use cavities opportunistically, then the distance between the final night location and following day location would be shorter than the average distance between the day location and all the night locations. In contrast, if pacas selectively return to specific cavities, then we would expect the distance between the final night location and following day location to be longer than or similar to the average distance between the day location and all the night fixes. Additionally, for each paca, we calculated the mean distance between closest neighboring pairs of cavities (shortest distances between a cavity and the nearest neighboring cavity) and compared between males and females using a Student’s t test.
We expected our observations of unique resting locations associated with a paca to eventually asymptote with monitoring effort (number of day locations recorded), on the assumption that home ranges contain a fixed number of suitable cavities. For each paca, we plotted the number of unique resting locations against the number of days for which day locations were recorded, to assess whether we could reliably estimate the number of resting locations used by the paca across its home range.
To assess the rate at which pacas alternate between resting locations, we calculated a ratio for each paca of the number of resting sites, R, to the number of day locations obtained, D. A value of 1 indicates that each day location occupied a different resting location, and a value close to 0 indicates re-use of the same resting site or sites.
Waterholes
We assessed the extent to which the paca ranges included access to water and its proximity to resting day locations by mapping the distribution of water holes and waterways within the study area in relation to home ranges, core ranges, and resting day locations. We tested whether the distance between used cavities and water sources differed between males and females using a Student’s t test.
All statistical tests were carried out using (R Core Team 2012).
Results
Paca home range sizes appeared to achieve an asymptote at ~ 125 locations based on area accumulation curves (AACs) (Fig. 1). MCPs of males ranged from 87.5 to 204.9 ha (X = 134.7 ± 43.1 SD, N = 4) and MCPs of females from 50.7 to 86.7 ha (X = 68.7 ± 18 SD, N = 2) (see Gutierrez et al. 2016 for details). For two males (M2 and M4) AACs did not plateau during the study duration, and therefore, these estimates, although the largest home ranges in the sample, should be considered conservative.
Area accumulation curves for six pacas radio-tracked in Central Belize, using bootstraps repeated 100 times at every step in a stepwise logarithmic progression. The solid trace is the average area accumulation, and the dotted and dashed traces are the 5 and 10% ranges of the accumulation. The horizontal solid line above each curve shows the 100% MCP
Static interaction: overlap between individuals
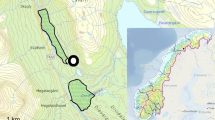
None of the simultaneously tracked pacas occupied exclusive home ranges or exclusive core ranges, and male pacas overlapped with the entire range of female F2 (Figs. 2 and 3). Core area overlap was low between all pairs of individuals, despite extensive home range overlap between some pairs (Table 1, Figs. 2 and 3). A male-female pair (M4-F2) had the least overlap, and a male-male pair had the greatest overlap (M2-M3, Table 1). However, home range overlap did not differ detectably between male-male pairs and male-female pairs (UDIO95: t = −0.138, UDOI50: t = −0.684, P > 0.5 and N = 7 for both). The 50% KHR home range cores overlapped less than the 95% home ranges (paired t-test: mean UDOI50 = 0.06 ± 0.05; mean UDOI95 = 0.38 ± 0.24; t = 4.67, P < 0.01, N = 7), potentially indicating a greater degree of exclusivity at the core of the range.
Home ranges (95% KHR) of simultaneously radio-tracked pacas in Central Belize. a Male, M1 (24 June 2010 to 6 January 2011) and female, F1 (24 June to 27 July 2010). b Female, F2 (2 January to 18 August 2011); male, M2 (7 June 2011 to 17 February 2012); male, M3 (8 June to 29 September 2011); male, M4 (10 June 2011 to 17 February 2012)
Core areas (50% KHR) of simultaneously radio-tracked pacas in Central Belize. a Male paca (M1, tracked: 24 June 2010 to 6 January 2011) and female paca (F1, tracked 24 June to 27 July 2010). b Female (F2, tracked 2 January 2011 to 18 August 11), male (M2, tracked 7 June 2011 to 17 February 2012), male (M3, tracked 8 June to 29 September 2011), male (M4, tracked 10 June 2011 to 17 February 12)
Static interaction: shifts in individuals’ ranges
The home ranges of males M1 and M3 shifted during the wet season (Fig. 4). Male M1 almost completely changed its range (UDOI95 = 0.06, first temporal monitoring period = 24 June to 6 September 2010, N = 49, second temporal monitoring period = 7 September 2010 to 6 January 2011, N = 21). Male M3 expanded its range by approximately 50% (UDOI95 = 0.53, first temporal monitoring period = 8 June to 20 July 2011, N = 22, second temporal monitoring period = 26 July to 29 September 2011, N = 20). In contrast, the home ranges of two of the other pacas remained relatively stable (F2; UDOI95 = 0.88, first temporal monitoring period = 2 January to 9 June 2011, N = 20, second temporal monitoring period = 10 June to 18 August 2011, N = 29; M2; UDOI95 = 0.76, first temporal monitoring period = 7 June to 6 September 2011, N = 31, second temporal monitoring period = 7 September 2011 to 17 February 2012, N = 20).
Home ranges (95% KHR) of four pacas (three males and one female) radio-tracked in Central Belize, split temporally to visualize range shifts; dashed line indicates the home range in the first temporal period of monitoring (t1), and solid line represents the home range within the second temporal period of monitoring (t2). F2: t1 = 2 January to 9 June 2011, t2 = 10 June to 18 August 2011; M1: t1 = 24 June to 6 September 2010, t2 = 7 September 2010 to 6 January 2011; M2: t1 = 7 June to 6 September 2011, t2 = 7 September 2011 to 17 February 2012; M3: t1 = 8 June to 20 July 2011, t2 = 26 July to 29 September 2011
Dynamic interaction
The three measures of dynamic interaction were broadly congruent. Overall, the measures indicated attraction between one male-female pair (M1-F1), a possible case of attraction, and two possible cases of avoidance between three male-male pairs (M2-M3, M3-M4, and M2-M4, respectively), and indifference between the remaining three male-female pairs (Table 2).
The distance between pairs of pacas through time revealed a possible mating event between F2-M3, who were < 5 m away from each other when simultaneously radio-tracked on 7 July 2011, and who were observed to be behaving and vocalizing in a manner that would suggest mating was occurring. At this time, the two other males (M2 and M4) were the furthest distance from the female for any period (M2 = 1547 m and M4 = 1311 m) even though their core areas overlapped with the female.
Cavity use
Day locations were recorded on 57 days, and we identified 38 distinct resting locations across the six pacas (Table 3). Because male M1 shifted his home range (and core area) almost completely (Fig. 4), we treated these as separate ranges when analyzing cavity use. For all pacas except one male, M4, all resting day locations were within the individuals’ nighttime home ranges, with the majority in the core areas (% in core areas: mean ± SD = 63 ± 21, N = 7 ranges; Table 3, Fig. 5). The percent of day locations occurring within core areas did not differ between the sexes (% day locations in core area, mean ± SD: male = 65 ± 24, N = 5 ranges; female = 59 ± 12, N = 2 ranges; t = − 0.44, df = 4, P > 0.5).
The average distance of the final night location to the corresponding resting location the following day was shorter compared to the average distance between all night locations and resting locations (paired t test: final night locations mean ± SD = 254 ± 150 m, N = 20; all night locations mean ± SD = 364 ± 106 m, N = 20, t = −3.191, df = 19, P < 0.005), suggesting that the pacas were using cavities opportunistically rather than returning to specific cavity sites at the end of their period of nocturnal activity. The distance of a paca resting location to the next closest cavity that it used was on average lower for females than for males, although this was not significantly different between males and females (mean distance ± SD: males 251 ± 147 m, N = 30; females 157 ± 113 m, N = 10, t = 1.836, df = 38, P > 0.05).
We monitored the pacas’ resting day locations with monitoring efforts ranging from 7 to 31 search days per paca (mean ± SD = 15 ± 9.0 days, N = 6 pacas) over periods ranging from 8 to 228 days per paca (mean ± SD = 127 ± 80 days, N = 6 pacas). The number of distinct resting sites ranged from 4, by female F2 who was monitored on 17 days over 228 days, to 12, by male M1, who was monitored on 31 days over a period of 168 days (Table 3). However, M1 shifted his home range and core area during this time (Fig. 4), using eight cavities in the first range and four in the second range. Female F1, who was monitored for 7 days over a period of 8 days, occupied six different burrows during this time, indicating a high rate of turnover between cavity sites (R/D ratio = 0.9, Table 3). Overall, we documented an average of 5.7 cavities per paca range (SD = 1.5, range = 4 to 8, N = 7 paca ranges). However, the number of unique resting locations identified increased with the monitoring effort for all pacas, and reached an asymptote for only one (female, F2, asymptote = 4 resting locations after six search days; see Supplementary material). For the other five pacas, there was no evidence that the number of resting locations identified approached an asymptote within the time that we monitored them. These should therefore be considered as conservative estimates (see Supplementary material).
For the pacas that we tracked simultaneously, we found no evidence of co-occupation of the same resting location at the same time. We found evidence of different individuals using the same resting location (albeit on different days) on two occasions only: female (F2) used a location previously used by a male (M1) who had abandoned the area ~ 4 months previously, and a location used 6 months earlier by a female (F1), who was no longer alive.
Waterholes
The home ranges of all pacas (and core areas of five pacas) overlapped with waterholes (Fig. 5). One male (M2) overlapped with all five waterholes available in the study area, while the other paca ranges each spanned at least four of the waterholes. One male (M1) shifted his range away from the waterholes towards a creek as it filled with water during the progression of the wet season. Female resting day locations were located closer to waterholes compared to those used by males (mean ± SD: male = 241 ± 175 m, N = 30, female = 133 ± 40 m, N = 10; t = 2.09, df = 38, P < 0.05).
Discussion
Our study of pacas inhabiting marginal habitat revealed extensive home range overlap between males and between the sexes. Male-male overlap was less extensive within core areas, while female core ranges were almost entirely occupied by one or more males. Males tended to avoid one another in areas where they overlapped, while females and males were more tolerant of one another. On average, pacas used at least six cavities within their home range, with the majority located in the core areas. On average, females occupied cavities that were closer together, and closer to water bodies, than those of males.
Gutierrez et al. 2016 indicated that the ranges of the pacas in this study were 20–100× larger compared to ranges reported in the literature for other paca studies. Our analysis shows that these are conservative, as the two largest male ranges did not reach an asymptote during the study. The smaller ranges published elsewhere may be associated with more productive habitats, the population existing at carrying capacity, or an artifact of small sample sizes and/or shorter monitoring periods (ranges of 2.3 to 3.4 ha in primary broadleaf forest in Costa Rica and on Barro Colorado Island, Panama; Beck-King et al. 1999; Marcus 1984 respectively).
We know of only one other study that has tracked multiple males and females simultaneously (Marcus 1984). The pacas in the moist broadleaf forest habitat of Barro Colorado Island showed exclusive, similar-sized ranges of pairs of males and females. In contrast, our study found that male ranges were larger than female ranges and overlapped extensively with same sex conspecifics. Although we were unable to study female-female overlap, we detected extensive home range overlap for male-male and male-female pairs, and no evidence of ranges exclusive to male-female pairs as documented by Marcus (1984). Male-male overlap was less extensive within core areas, while female core ranges were almost entirely occupied by one or more males. Interactions between pacas varied with sex. Males were generally intolerant of one another during nocturnal activity, with two pairs weakly avoiding one another, while the third male-male pair displayed weak attraction, potentially an artifact of both being attracted to the same resource (e.g., a female or food). In contrast, male-female pairs were generally tolerant of one another, with one pair showing attraction and the other three pairs displaying indifference. This suggests a system in which males and females do not defend food resources from each other during nocturnal activity. From the limited number of pacas sampled, the system appears to be one in which males are distributed according to females, the larger ranges of multiple males overlapping with each other and with the smaller female ranges. The larger ranges of males than females and the extensive overlap between conspecifics suggest a polygamous or promiscuous mating system.
Pacas maintained their core areas more exclusively than their home ranges, with the distribution of use overlapping < 13% between any given pair of core areas. With the exception of one paca, ≥ 50% of the resting day locations were located within the core areas of the respective individuals. Individual pacas used these resting sites exclusively. Although pacas in our study area appeared to select their resting locations somewhat opportunistically, we detected repeated use of the same sites. Overall, these data suggest that pacas use cavities within their core range but move often, and apparently opportunistically, between the available sites. It is not clear what drives the shift between different cavity sites. They could be excluded from cavities by other competing species (e.g., Figueroa-de-León et al. 2016), or they may move frequently to avoid detection by predators, or in response to environmental changes (e.g., prevailing weather conditions) and the condition, quality or permanence of the cavity. Observations in the field revealed that some of the resting sites were relatively shallow and exposed, unlike the complex cavity systems described elsewhere (e.g., Aquino et al. 2009). Complex “higher quality” cavity systems may be more common in habitat with well-drained soils. Using camera traps in the Lacandon rainforest in Mexico, Figueroa-de-León et al. (2016) found that the presence of pacas at cavity sites and the permanency of cavity use depended on the type of cavity, permanency and protection against collapse in the wet season. The poor drainage of our study area, where cavities are prone to flooding or collapse in the wet season, may necessitate that pacas defend multiple locations as contingency. Thus, in areas where high-quality cavities are limited, competition for these sites may be stronger between females than males.
Figueroa-de-León et al. (2016) also found that the presence of pacas at cavity sites and the permanency of cavity use depended on proximity to water. Aquino et al. (2009) suggest that resting sites > 100 m from water bodies would be too far to out run a predator, and under these circumstances, one predator avoidance strategy would be to maintain multiple locations in close proximity to one another which they may escape between, confusing the predator. In our study, both male and female resting locations were on average further than 100 m to water bodies; however, female cavities were on average closer to one another than were male cavities. Potentially, the selection pressure to rest in close proximity to water, and to have nearby alternative cavities, is greater for females with vulnerable young than for males, who may be more willing to move away from water sources and suitable cavities in search of mates.
In primary broadleaf forest, Marcus (1984) tracked 22 pacas and reported use of single cavities by individuals, while Beck-King et al. (1999) estimated an average of 3.5 cavities per paca in Costa Rica. The low estimates of cavity use in both studies may reflect the presence and use of cavities of higher quality in the primary broadleaf forest compared with the marginal habitat of our study area where pacas used on average at least six cavities. Additionally, the low estimate by Beck-King et al. (1999) may be an artifact of the low sample size (two individuals tracked) and short monitoring period. Like Marcus (1984) and Beck-King et al. (1999), we found no evidence of pacas using each other’s resting locations; however Figueroa-de-León et al. (2016) detected 21 adults and 9 young across 16 monitored cavities, implying a degree of temporal sharing. Females were either alone or with their young, and males used the same cavities on rare occasions for short periods only (1 day) when the female and young were absent (Figueroa-de-León, personal communication). The number and exclusivity of cavity use appears to vary between sites, potentially influenced by a suite of biotic and abiotic factors (e.g., habitat structure, competitors, predators, and weather conditions).
The habit of resting in cavities during the day makes pacas an easy, predictable target for hunters throughout Latin America, who traditionally use dogs to locate resting sites and chase or dig them out (e.g., El Bizri et al. 2016). This makes pacas particularly vulnerable to hunters in habitats that have a low availability of complex, high-quality cavity systems. The use of multiple cavities by pacas may reflect the intensity of hunting and predation within an area, with frequent disturbance necessitating frequent shifts to alternate refuge locations. Hunting of pacas is common throughout the CBC, as is the presence of natural predators such as jaguars and pumas (Urbina et al., unpublished data, Foster et al., in prep). In some environments at least, cavities may be a limited resource worth monitoring for potential paca management. For example, Figueroa-de-León et al. (2016) recommend the conservation of cavities around rural communities to support paca populations as prey for the local people and predators. Furthermore, reliable estimates of the average number of cavities used per paca and the degree of sharing/exclusivity could be used as a method for estimating paca density from counts of active cavities (e.g., Beck-King et al. 1999; Harmsen and Foster unpublished data).
The social system and hence the spatial distribution of pacas may be much more plastic than previously assumed, responding to biotic and abiotic factors such as habitat structure, cavity availability, permanence and complexity, water availability, predation/hunting pressure, and paca population density. Understanding in detail how paca spatial distribution and social structure are influenced by these factors across different landscapes will aid density assessments and improve assessments of sustainable harvest rates.
References
Altrichter M (2001) The importance of wild mammals in the diet of the populations of Osa Peninsula, Costa Rica. Rev Mex Mastozoologia 4:99–107
Aquino R, Gil D, Pezo E (2009) Ecological aspects and hunting sustainability of paca (Cuniculus paca) in the Itaya river basin, Peruvian Amazonia. Rev Peru Biol 16:67–72
Aquino R, Meléndez GM, Pezo E, Gil D (2012) Types and forms of sleeping dens of pacas (Cuniculus paca) in the upper Itaya river basin. Rev Peru Biol 19:27–34
Ausband DE, Stansbury CR, Stenglein JL, Struthers JL, Ad L, Waits P (2015) Recruitment in a social carnivore before and after harvest. Anim Conserv 18(5):415–423. https://doi.org/10.1111/acv.12187
Beck-King H, von Helversen O, Beck-King R (1999) Home range, population density and food resources of Agouti paca (Rodentia: Agoutidae) in Costa Rica: a study using alternative methods. Biotropica 31(4):675–685. https://doi.org/10.1111/j.1744-7429.1999.tb00417.x
Benhamou S, Valeix M, Chamaille-Jammes S, Macdonald DW, Loveridge AJ (2014) Movement-based analysis of interactions in African lions. Anim Behav 90:171–180. https://doi.org/10.1016/j.anbehav.2014.01.030
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197(3-4):516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Collet, S. 1981. Population characteristics of Agouti paca (Rodentia) in Colombia. Publications of the Museum, Biological Series, 5:485–602. Michigan State University, Lansing, Michigan
Coltman DW, O’Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M (2003) Undesirable evolutionary consequences of trophy hunting. Nature 426(6967):655–658. https://doi.org/10.1038/nature02177
Doncaster CP (1990) Non-parametric estimates of interaction from radio-tracking data. J Theor Biol 143(4):431–443. https://doi.org/10.1016/S0022-5193(05)80020-7
Doncaster, C. P., R.J. Foster, and B.J. Harmsen. 2012. Belize large-mammal corridor project, Darwin Initiative Final Report, University of Southampton, UK
Dubost G, Henry O (2006) Comparison of diets of the acouchy, agouti, paca, the three largest terrestrial rodents of French Guianan forests. J Trop Ecol 22(06):641–651. https://doi.org/10.1017/S0266467406003440
El Bizri H, da Silva Araújo LW, da Silva Araújo W, Maranhão L, Valsecchi J (2016) Turning the game around for conservation: using traditional hunting knowledge to improve the capture efficiency of Amazon lowland pacas. Wildl Biol 22(1):1–6. https://doi.org/10.2981/wlb.00127
Emmons, L., E. Vieira, D. Queirolo, R. Samudio Jr. 2016. Cuniculus paca. The IUCN Red List of Threatened Species v. 2017.2. https://www.iucnredlist.org [accessed 17 oct 2017]
Escamilla A, Sanvicente M, Sosa M, Galindo-Leal C (2000) Habitat mosaic, wildlife availability, and hunting in the tropical forest of Calakmul, Mexico. Conserv Biol 14(6):1592–1601. https://doi.org/10.1046/j.1523-1739.2000.99069.x
Fa JE, Peres CA (2001) Game vertebrate extraction in African and Neotropical forests: an intercontinental comparison. In: Reynolds JD, Mace GM, Redford KH, Robinson JG (eds) Conservation of exploited species. Cambridge University Press, Cambridge, pp 203–241
Fieberg J, Kochanny CO (2005) Quantification of home range overlap: the importance of the utilization distribution. J Wildl Manag 69(4):1346–1359
Figueroa-de-León A, Naranjo EJ, Perales H, Santos-Moreno A, Consuelo L (2016) Cavity occupancy by lowland paca (Cuniculus paca) in the acandon rainforest, Chiapas, Mexico. Trop Conserv Sci 9(1):246–263. https://doi.org/10.1177/194008291600900113
Foster RJ, Harmsen BJ, Macdonald DW, Collins J, Urbina Y, Garcia R, Doncaster CP (2016) Wild meat: a shared resource amongst people and predators. Oryx 50(01):63–75. https://doi.org/10.1017/S003060531400060X
Fritz H, Loison A (2006) Large herbivores across biomes. In: Danell K, Bergström R, Duncan P, Pastor J (eds) Large herbivore ecology, ecosystem dynamics and conservation. Cambridge University Press, Cambridge, pp 19–48. https://doi.org/10.1017/CBO9780511617461.003
Gordon IJ, Acevedo-Whitehouse K, Altwegg R, Garner TWJ, Gompper ME, Katzner TE, Pettorelli N, Redpath S (2012) What the ‘food security’ agenda means for animal conservation in terrestrial ecosystems. Anim Conserv 15(2):115–116. https://doi.org/10.1111/j.1469-1795.2012.00541.x
Gutierrez SM, Harmsen BJ, Doncaster CP, Kay E, Foster RJ (2016) Ranging behavior and habitat selection of pacas (Cuniculus paca) in central Belize. J Mammal 98:542–550
Harmsen BJ, Foster RJ, Silver SC, Ostro LET, Doncaster CP (2010) Jaguar and puma activity patterns in relation to their main prey. Mamm Biol 76:320–324
Herrera EA (2016) Caviomorphs as models for the evolution of mating systems in mammals. In: Ebensperger LA, Hayes LD (eds) Sociobiology of caviomorph rodents, an integrative approach. Wiley Blackwell, Oxford, pp 253–272. https://doi.org/10.1002/9781118846506.ch10
Kay E, Dickerson A, Urbina Y, Lizama D, Correa E, Cruz F, Garcia R, Thompson R, Williams L, Quintana R, Young J, Andrewin-Bohn J, Cawich V, Joseph R, Humes S, Mai A (2015) Central Belize corridor: conservation action plan. University of Belize, Belmopan
Kenward RE, Marcstrom V, Karlbom M (1993) Post-nestling behaviour in goshawks, Accipiter gentilis: II. Sex differences in sociality and nest-switching. Anim Behav 46(2):371–378. https://doi.org/10.1006/anbe.1993.1199
Koster J (2008) The impact of hunting with dogs on wildlife harvests in the Bosawas Reserves, Nicaragua. Environ Conserv 3:211–220
Kranstauber, B. and M. Smolla. 2016. Move: visualizing and analyzing animal track data. R package version 1.6.541. Available at: https://cran.rproject.org/web/packages/move/move.pdf
Laska M, Baltazar JML, Luna ER (2003) Food preferences and nutrient composition in captive pacas, Agouti pacas. Mamm Biol 68(1):31–41. https://doi.org/10.1078/1616-5047-00059
Lone K, Loe LE, Meisingset EL, Stamnes I, Mysterud A (2015) An adaptive behavioural response to hunting: surviving male red deer shift habitat at the onset of the hunting season. Anim Behav 102:127–138. https://doi.org/10.1016/j.anbehav.2015.01.012
Long JA, Nelson TA, Webb SL, Gee K (2014) A critical examination of indices of dynamic interaction for wildlife telemetry studies. J Anim Ecol 83(5):1216–1233. https://doi.org/10.1111/1365-2656.12198
Macdonald, G. 2013. “Cuniculus paca” (On-line), Animal diversity web. Accessed October 16, 2017 at http://animaldiversity.org/accounts/Cuniculus_paca/
Marcus, M. 1984. Behavioral ecology of paca (Agouti paca) on Barro Colorado Island, Panama. M.S. thesis, University of Maine. Maine United States of America
Milner JS, Nilsen EB, Andreassen HP (2007) Demographic side effects of selective hunting in ungulates and carnivores. Conserv Biol 21(1):36–47. https://doi.org/10.1111/j.1523-1739.2006.00591.x
Mysterud A (2014) Effects of selective harvesting on ungulate populations. In: Putman R, Apollonio M (eds) Behaviour and management of European ungulates. Whittles Publishing, Caithness, pp 124–147
Ojasti, J. 1996. Wildlife utilization in Latin Amerca: current situation and prospects for sustainable management. FAO Conservation Guide 25, UN, Rome
Ordiz A, Stoen OG, Saebo S, Kindberg J, Delibes M, Swenson JE (2012) Do bears know they are being hunted? Biol Conserv 152:21–28. https://doi.org/10.1016/j.biocon.2012.04.006
Pérez EM (1992) Agouti paca. Mamm Species 404:1–7
Proffitt KM, Grigg JL, Garrott RA, Hamlin KL, Cunningham J, Gude JA, Jourdonnais C (2010) Changes in elk resource selection and distributions associated with a late-season elk hunt. J Wildl Manag 74(2):210–218. https://doi.org/10.2193/2008-593
Quaglietta, L., V. C. Fonseca, , A. Mira, and L. Boitani. 2014. Sociospatial organization of a solitary carnivore, the Eurasian otter (Lutra lutra). J Mammal 95:140–150, 1, DOI: https://doi.org/10.1644/13-MAMM-A-073.1
R Core Team. 2012. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, ISBN 3–900051–07-0, URL http://www.R-project.org/
Redford KH (1999) The empty forest. Bioscience 42:412–422
Reider KE, Carson WP, Donelly MA (2013) Effects of collared peccary exclusion on leaf litter amphibians and reptiles in Neotropical wet forest, cost Rica. Biol Conserv 163:90–98. https://doi.org/10.1016/j.biocon.2012.12.015
Smith DA (2005) Garden game: shifting cultivation, indigenous hunting and wildlife ecology in western Panama. Hum Ecol 33(4):505–537. https://doi.org/10.1007/s10745-005-5157-Y
Smythe, N., and O. Brown de Guanti. 1995. Domestication and husbandry of the paca (Agouti paca). http://www.fao.org/docrep/006/V4940S/V4940S00.HTM
Stoner DC, Wolfe ML, Choate DM (2006) Cougar exploitation levels in Utah: implications for demographic structure, population recovery, and metapopulation dynamics. J Wildl Manag 70(6):1588–1600
Stoner KE, Vulinec K, Wright SJ, Peres CA (2007) Hunting and plant community dynamics in tropical forests: a synthesis and future directions. Biotropica 39(3):385–392. https://doi.org/10.1111/j.1744-7429.2007.00291.x
Ulloa L, Rodriguez D, Sanchez P (1999) Movimientos y uso del tiempo y el espacio por una guartinaja (Agouti paca) en la Sierra Nevada De Santa Marta, Colombia. Rev Acad Colomb Cienc 23:687–694
Acknowledgements
We thank M. Brakeman and the many students and volunteers who assisted with data collection. This work is dedicated posthumously to our friend A. Ramos, whose field craft made the project possible. We gratefully acknowledge his vital support to the project and the countless hours of fieldwork he devoted to its completion.
Funding
This research was funded by the UK Darwin Initiative 17-012 and Panthera.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Emerson M. Vieira
Electronic supplementary material
Supplementary material
Increase in detection of unique day resting locations with monitoring effort for six pacas radio-tracked in the Central Belize Corridor. (TIFF 79 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Harmsen, B.J., Wooldridge, R.L., Gutierrez, S.M. et al. Spatial and temporal interactions of free-ranging pacas (Cuniculus paca). Mamm Res 63, 161–172 (2018). https://doi.org/10.1007/s13364-017-0350-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-017-0350-0