Abstract
In the cephalopod subclass Coleoidea, several homology problems exist, mainly owing to unsolved phylogenetic relationships between decabrachian orders. The present contribution reviews the “similarity” of the gladius, the chitinous shell rudiment in the dorsal mantle that provides rigid attachment sites for the locomotory-relevant musculature. As a secretion product of the shell sac epithelium as well as in the light of a common three-layered construction, both the octobrachian and the decabrachian gladius types most probably represent homologues with identical developmental mechanisms; “similarities” in gladius shapes in unrelated lineages therefore should be considered as the result of parallelism. Ultrastructural comparisons with Mesozoic coleoids suggest that an organic gladius is actually embedded in every proostracum-bearing phragmocone. It is therefore generally accepted that a gladius evolved through decalcification of a proostracum-bearing phragmocone. The character “gladius” accordingly represents a plesiomorphy within pro-ostracum-bearing coleoids. Whereas the gladius of Vampyroteuthis as well as the octopod fin supports indirectly derived from phragmoteuthid-like phragmocone via Mesozoic gladius types, the decabrachian gladius types can morphogenetically be linked with various ancestral groups (Belemnitida, Diplobelida, Groenlandibelidae, Vasseuria, Belosepiella). Experimental decalcification of a sepiid cuttlebone demonstrates furthermore that a gladius might have also evolved from a secondarily proostracum-less phragmocone. Life styles and habitats of living and Mesozoic gladius-bearing octobrachians are finally discussed in the light of our conclusions.
Similar content being viewed by others
Introduction
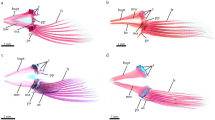
The gladius (English “pen”; French “plume”; German “Schulp”) is a chitinous, spatulate structure located in the dorsal midline of the body of coleoid cephalopods. This sturdy “backbone” typically occupies the full length of the dorsal mantle and provides attachment of various locomotory-relevant musculatures, which makes the gladius one of the key innovations responsible for the most powerful mode of jet-propulsion among cephalopods. A gladius occurs in Recent Decabrachia (Loliginida, Oegopsida, Bathyteuthoidea, Sepiolida, and Idiosepiidae; Fig. 1a–c) as well as in the deep sea vampire squid, Vampyroteuthis (Fig. 1d). In dependence on their morphogenetic derivation, the octobrachian fin supports can furthermore be seen as gladius vestiges (Haas 2002; Bizikov 2004).
In the past, gladius-bearing forms have often been lumped together (“Chondrophora” Gray 1849, “Incamerophora” Khromov 1990). Workers accordingly assumed the gladius to be a unique development (e.g. Clarke 1988). Authorities as Naef (1922) and Jeletzky (1966) distinguished between gladius-bearing “Teuthida” and “Sepioidea”, which include forms with a calcareous shell (Sepiida, Spirulida) as well as forms with unmineralized shell remains of gladius-like appearances (Sepiolida, Idiosepiidae). Later, during the era of strict phylogenetic principles and molecular cladistics, it became evident that Vampyroteuthis belongs to the octobrachian branch and also the taxon “Teuthida” has split up in (sometimes many) isolated evolutionary lineages (e.g. Berthold and Engeser 1987; Haas 1997, 2002; Carlini et al. 2000; Lindgren et al. 2004; Strugnell and Nishiguchi 2007; Bizikov 2008; Strugnell et al. 2009). The new divergence patterns have left some unsatisfactory features, particularly regarding their impact on the formerly assumed homology of the gladius. Few authors, who have tried to approach this homology problem, have unfortunately avoided an in-depth argumentation (e.g. Young et al. 1998; Lindgren 2010: p. 85). Since a clear distinction between homology and homoplasy is essential for interpretations on the evolution of organismic groups and their characters, it is the purpose of the present contribution to recapitulate the causes of this homology problem and to summarize available information from the literature about the general morphogenetic origin of a gladius. We will explain why convergent traits are unlikely and why some gladius types represent homologues and others parallelisms.
Although Bizikov (2008) has recognized ten different gladius morphotypes, we prefer in the present context to formally distinguish between only six different gladius types representative for the Recent coleoid taxa Loliginida (=Myopsida), Oegopsida, Bathyteuthoidea, Sepiolida, Idiosepiidae, and Vampyromorpha. Later in the discussion, we will additionally refer to three fossil gladius types.
Discussion
Inconsistencies regarding the (non)homologue nature of Recent gladius types certainly arise from different approaches, namely interpretations before (a priori) and after (a posteriori) the construction of phylogenetic trees.
A priori
Regarding the homology criterion of position (Remane 1952), each gladius type is formed by the shell sac epithelium and located in the dorsal midline of the mantle. Hence, with respect to its formation site, namely the shell gland, it does not appear arbitrary to consider all gladiuses a priori as homologous.
In their extensive studies on modern gladiuses, both Toll (1982, 1988) and Bizikov (1996, 2004, 2008) have shown that gladiuses are generally composed of three principle shell layers (note the general construction of the idiosepiid gladius is still poorly studied). The intermediate layer is lamellar and composed of hard chitinous substance. This growth increments-bearing layer is present along the entire length of the gladius, in contrast to the inner and outer layer, both of which are mostly restricted to posterior gladius parts and composed of a more resilient chitinous material (Arkhipkin et al. 2012). Despite modifications and reductions of the inner and outer layer, this general construction can be found in all gladius types (to a lesser extent in octopod gladius vestiges) confirming—with respect to Remane´s criterion of special quality—the assumed homology.
A posteriori
The following argumentation is based on the phylogenetic tree illustrated in Fig. 2a. Its poorly resolved divergence patterns within the Decabrachia is in accordance with many previous proposals (e.g. Boletzky 1999; Young et al. 1998). The divergence pattern includes three well-established presumptions: (1) the presence of a calcareous and chambered shell in Recent Sepiida and Spirula represents a plesiomorphy within the Cephalopoda (e.g. Young et al. 1998; Kröger et al. 2011; Doguzhaeva and Dunca this volume), (2) Octobrachia (=Vampyropoda) and Decabrachia represent monophyla, and (3) Vampyromorpha is the sister of Octopoda (Cirrata + Incirrata). In the light of these three presumptions, it becomes clear that the presence of calcareous chambered shells in the Decabrachia (Sepiida, Spirula) indicates an independent evolution of an unmineralized gladius in the octobrachian and the decabrachian lineage. As the polytomy in Fig. 2a indicates, the divergence pattern within the Decabrachia is still under discussion. Theoretically, a gladius might have been emerged up to five times only in the Decabrachia.
Phylogeny of Coleoidea without (a) and with (b) fossil representatives. Indicated are only apomorphies and plesiomorphies related to the evolution of a gladius. The topology in b is based on Kröger et al. (2011) and Fuchs et al. (2013, in press). Stratigraphic occurrences or divergence times have been discarded
As a preliminary result, the thorough application of homology criteria suggest a priori a homology between the different gladius types, whereas a posteriori considerations rather point to homoplasious (convergent or parallel) traits. A closer look at the morphogenetic origin of the gladius helps to better resolve this dilemma.
General morphogenetic origin of a gladius
Experts widely agree that a gladius evolved through decalcification of a proostracum-bearing phragmocone (e.g. Jeletzky 1966; Toll 1998; Haas 2002; Fuchs 2006b, c; Arkhipkin et al. 2012; Bizikov and Toll 2015). A proostracum describes the dorsal forward projection of the straight (orthoconic to breviconic) phragmocones of extinct Phragmoteuthida, Belemnitida, Diplobelida, and Groenlandibelidae (Fig. 3a–d). Bizikov (1996, 2008), for that reason, subdivides the gladius in the proostracum and the posterior cone, the latter of which corresponds to the phragmocone of ancestral coleoids. By comparison, the more primitive coleoid orders Hematitida, Donovaniconida, and Aulacoceratida exhibit a tubular terminal chamber without any evidence of a distinctly forward projecting proostracum (Fig. 3e). The ventral reduction of the terminal chamber (“body chamber”) represents a seminal event in the evolution of Coleoidea as it gives way to the development of a liberated muscular mantle, which can now attach to the lateral margins of the dorsal proostracum (e.g. Jeletzky 1966; Fuchs et al. in press). The taxa Phragmoteuthida (Permian?, Triassic-Lower Jurassic), Belemnitida (Upper Triassic-Upper Cretaceous), Diplobelida (Lower Jurassic-Upper Cretaceous), and Groenlandibelidae (Upper Cretaceous) represent a stratigraphical succession that suggests a gradual decrease of the proostracum width (Fig. 2b). This morphological chain can be seen as an evolutionary trend towards a significantly extended muscular mantle and thus towards more powerful mantle contractions (Naef 1922; Engeser and Bandel 1988; Kröger et al. 2011; Fuchs et al. 2013). Palaeontological evidence for multiple independent formations of a proostracum do—to the authors best knowledge—not exist; a strong argument against the alleged distinction between “Paleocoleoidea” (=proostracum-less and proostracum-bearing Belemnoidea) and “Neocoleoidea” (=extant lineages of Coleoidea), which would afford at least two independent origins of a proostracum (compare Young et al. 1998; Haas 2002, 2003; Fuchs et al. 2010; Kröger et al. 2011).
Comparative morphology of coleoid phragmocones (external and internal shell features are neglected). With dorsal proostracum (a Phragmoteuthida; b Belemnitida; c Diplobelida; d Groenlandibelidae); e without dorsal proostracum (e.g. Donovaniconida); f with reduced ventral wall (Sepiidae). Please note that the relative proostracum length of Groenlandibelidae is still unknown
The sepiid cuttlebone has occasionally been considered to bear a proostracum (Fig. 3f). However, its evolutionary shell transformations via coiled phragmocones without proostracum (Belosaepia, Ceratisaepia) clearly point to a secondarily proostracum-like appearance of the dorsal shield of the cuttlebone (Fig. 3f; Naef 1922; Barskov 1973; Young et al. 1998; Haas 2003). Similarly, Cenozoic spirulids have sometimes been reconstructed with a proostracum that has never been confirmed (Fuchs 2006a, b).
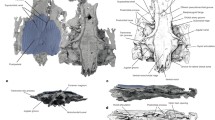
Doguzhaeva et al. (2003), Fuchs et al. (2007b), and Doguzhaeva and Summesberger (2012) have ascertained a lamello-organic construction of the shell layer that composes the proostracum (Fig. 4a). The organic proostracal layer is hence sandwiched by mineralized inner (conothecal) and outer (rostral) layers. Fuchs et al. (2007b) and Arkhipkin et al. (2012) have proposed—on the basis of its organic composition—that the proostracal layer corresponds to the molluscan periostracum. Doguzhaeva and Mutvei (2003) and Doguzhaeva and Mutvei (2006) have additionally observed ultrastructural similarities between the lamination of the proostracum of the belemnitid Belemnotheutis and the gladius of fossil and Recent squids (Fig. 4b, c). Jeletzky (1966) and recently Fuchs et al. (2012, 2013) described a very narrow, rod-like proostracum in Late Cretaceous groenlandibelids, which is likewise composed of multi-laminated shell material.
Multi-laminated ultrastructure of a belemnitid proostracum (a), octobrachian gladius (b), and a Recent decabrachian gladius (c). a Pachyteuthis sp., Belemnitina, Bathonian, Saratov, Russia; scale bar 100 µm. b Teudopsis buneli, Teudopseina, Toarcian, Luxembourg, scale bar 200 µm. c Todarodes pacificus, Ommastrephidae, Oegopsida, Japan, scale bar 10 µm
With respect to the latter structural consistency as well as the presumed common morphogenetic origin, it is reasonable to conclude that the gladius corresponds either to the proostracal (=periostracal) shell layer or—taking organic matrices of the mineralized layers into account—more generally to the organic components of a proostracum-bearing phragmocone (e.g. Jeletzky 1966: p. 47; Toll 1988; Young and Vecchione 1996). “As most calcareous coleoid shells probably have a gladius buried within the structure (…), evolution of a gladius is probably not a difficult step.”, as Young et al. (1998: p. 409) hit the nail on the head. In other words, all proostracum-bearing coleoids are—in a strict sense—gladius-bearing coleoids (the possibility that proostracum-less coleoids can also carry a gladius in their phragmocone will be discussed below). All gladius types accordingly represent homologous features inherited from an ancestral group with a distinctly forward projecting proostracum. The latter aspect particularly accommodates the common definition, where homology is the similarity of characters that has been inherited from a most recent ancestor (e.g. Patterson 1982; Hall 2007). The character state “gladius present” is for that reason actually plesiomorphic within pro-ostracum-bearing Coleoidea. Decabrachia (Loliginida, Oegopsida, Bathyteuthoidea, Sepiolida, Idiosepiida) and Octobrachia have consequently not “evolved” a gladius; they have rather “exposed” the gladius through the lost ability to deposit calcified shell layers. Decalcification of a phragmocone is equivalent to the loss of a buoyancy device (Arkhipkin et al. 2012). This can be deduced from hydrostatical aspects on the one hand and from the absence of an unmineralized phragmocone in the fossil record on the other hand.
So, if Recent gladius types represent homologues with identical developmental mechanisms inherited from a most recent ancestor, it becomes clear that the similarity of gladiuses in different lineages can impossibly be the result of convergence, which is usually characterized by different developmental pathways of non-homologous characters. The iterative appearance of a similar gladius shape in phylogenetically distant branches is therefore with some certainty a matter of parallelism, the similarity of homologous characters arisen from independent evolution; in the present context from different phragmocone types.
Ideas on the origin of octobrachian and decabrachian gladiuses
Octobrachia
Until the mid 1980s, workers commonly assumed the Phragmoteuthida to be the root-stock of most gladius-bearing groups. Authors such as Jeletzky (1966), Donovan (1977) or Doyle et al. (1994) believed that (at least) the vampyromorph, loliginid, and oegopsid gladius is linked with the phragmoteuthid phragmocone via the three main types of Mesozoic gladiuses belonging to the Prototeuthina, Loligosepiina, and Teudopseina. A similar gladius shape led many authors to classify e.g. Late Jurassic genera Plesioteuthis (Prototeuthina, Fig. 5a) and Palaeloligo (Teudopseina, Fig. 5c) respectively as ommastrephid oegopsids and bathyteuthoids (see e.g. Naef 1922; Jeletzky 1966; Donovan 1977; Riegraf et al. 1998; Bizikov 2008; Strugnell et al. 2006; Donovan and Strugnell 2010). The latter authors have thus reconstructed a direct ancestry and have therefore homologized fossil and Recent teuthoid gladiuses. However, a significantly increased understanding of fossil anatomies (note plesioteuthids belong to the best-known fossil coleoids) have meanwhile corroborated the idea introduced by Bandel and Leich (1986) whereupon Mesozoic gladius-bearing coleoids exclusively belong to the octobrachian branch (for detailed arguments supporting octobrachian affiliations the reader is referred to Haas (2002), Klug et al. (2005, in press), Fuchs (2006a, c), Fuchs et al. (2007c), Fuchs and Larson (2011a, b), Kröger et al. (2011), Donovan and Fuchs (in press). In the present context, it is crucial to note that the mode of muscular mantle attachment is fundamentally different in fossil and Recent gladiuses (Fuchs et al. in press). Accordingly, the “similarity” between the gladius shapes of octobrachian Plesioteuthis (as well as closely related prototeuthid genera Senefelderiteuthis and Dorateuthis) and Ommastrephidae (Fig. 5f) on the one hand and Palaeololigo (as well as closely related teudopseid genera such as Rachiteuthis and Marekites) and bathyteuthoids (Fig. 5g) on the other hand is superficial and thus an excellent example of parallelism (between families belonging to different superorders).
3-d reconstructions of various gladius types and a sepiid cuttlebone. a–e Fossil; f–i Recent; a Plesioteuthis prisca (Tithonian; Prototeuthina); b Rachiteuthis donovani (Cenomanian, Teudopseina); c Palaeololigo oblonga (Tithonian, Teudopseina); d Parabelopeltis flexuosa (Toarcian, Loligosepiina); e Trachyteuthis nusplingensis (Kimmeridgian, Teudopseina); f Ommastrephes (Oegopsida) sp.; g Chtenopteryx (Bathyteuthoidea); h Vampyroteuthis infernalis (Vampyromorpha); i Sepia sp. (Sepiida). Dorsal aspects (except g)
In contrast to Palaeololigo, Late Cretaceous palaeololiginids Rachiteuthis and Marekites are typified by possessing a long and slender, arrow-like gladius quite similar to that in plesioteuthids (compare Fig. 5a, b). If this classification is correct, the “similarity” between the gladius shape of Late Cretaceous Rachiteuthis and Marekites on the one hand and Plesioteuthidae on the other represents an example of parallelism in families belonging to the same superorder, but different suborders.
Although we cannot exclude the possibility of parallelisms on lower taxonomic levels, a similar gladius shape (in connection with identical mantle attachment sites) displayed in Jurassic loligosepiids (e.g. Geopeltis, Parabelopeltis, Doryanthes, Mastigophora; Fig. 5d) and Recent Vampyroteuthis (Figs. 1d, 5h) are tentatively considered as a matter of direct ancestry (Fuchs and Weis 2008). The vampyromorph gladius type is hence an example of a highly conservative shell morphology.
An unusual case of parallelism can be recognized with the trachyteuthid gladius (Fig. 5e). It is characterized by the presence of granules on its dorsal surface very similar to those on the sepiid cuttlebones (Fig. 5i). The dorsal granulation combined with some resemblance in shape caused earlier workers to consider trachyteuthids (e.g. Trachyteuthis, Glyphiteuthis, Actinosepia) as early sepiids (e.g. Roger 1952; Donovan 1977; Khromov 1990; Doyle et al. 1994). Firstly, trachyteuthid gladiuses (as all Mesozoic gladiuses) are unmineralized and without any evidence of a chambered phragmocone (Fuchs et al. 2007a). Secondly, soft part morphologies clearly speak for octobrachian affinities (Donovan et al. 2003; Klug et al. 2005; Fuchs and Larson 2011a). Thirdly, as advocated by Haas (2002) and Bizikov (2004), the teudopseid gladius type (to which the trachyteuthid unambiguously belongs) represents the ideal ancestral form from which the octopod gladius vestiges has possibly evolved (see also Fuchs 2009). To conclude, the last common ancestor of trachyteuthids and sepiids can be found far back in the stem-lineage of proostracum-bearing coleoids. Nevertheless, since the Sepiida probably derived from proostracum-bearing belemnoids, the dorsal granulation as well as the similar shape can be interpreted as the result of parallel evolution.
To conclude, a direct derivation of Octobrachia from phragmoteuthid-like ancestors only explains the morphogenetic origin of the octobrachian gladius, but disintegrates all connecting links leading to the decabrachian gladius types.
Decabrachia
Phylogenetic approaches often suggest a multiple loss of calcification (hence, the exposure of a gladius) within crown decabrachians (Fig. 2a, b). We have four pre-Cenozoic pro-ostracum-bearing groups (Phragmoteuthida, Belemnitida, Diplobelida, Groenlandibelidae) from which the gladiuses of Oegopsida, Bathyteuthoidea, Loliginida, Sepiolida, and Idiosepiidae could have been derived either directly or indirectly.
Before we draw further conclusions we first need to clarify the question whether ancestral decabrachians possessed a proostracum in their body plan at all. This question previously posed by Fuchs (2006b) is based on an idea whereupon Decabrachia are directly linked with their bactritid ancestors via putative Carboniferous spirulids (see Doguzhaeva et al. 1999). If so, the rod-like “proostraca” of Late Cretaceous groenlandibelid spirulids (Groenlandibelus, Cyrtobelus, Naefia) might have evolved independently from the belemnoid types of Mesozoic proostraca. However, the existence of Carboniferous spirulids is in the eyes of the present authors doubtful. Fuchs et al. (2012, 2013) have recently found support for an origin of crown decabrachians within Late Jurassic/Early Cretaceous Diplobelida (as already assumed by Naef 1922), which is characterized by a comparatively narrow proostracum (Fig. 2b). Derivation of the loliginid and oegopsid types of gladius from a diplobelid or groenlandibelid proostracum appears conceivable (Fig. 3c, d). Alternative origins for decabrachian types of gladiuses have been proposed by Haas (1997, 2003), Fuchs (2006b), and Arkhipkin et al. (2012). Haas (1997, 2003) has introduced the idea whereupon the loligind gladius derived from Vasseuria, an Eocene proostracum-bearing coleoid, whose spirulid affinities are still obscure. Haas (1997, 2003) has furthermore put forward a phylogenetic link between Sepiolida and Eocene Belosepiella (likewise of uncertain phylogenetic affinities). Owing to similarities in conus characteristics (presence of rudimental septa and rostrum), Arkhipkin et al. (2012) have discussed deviation of oegopsid teuthoids from belemnites. The latter scenario would strongly impact the assumption of a monophyletic origin of the Decabrachia, but an early divergence of Oegopsida is congruent with many topologies (e.g. Haas 2003; Carlini et al. 2000; Strugnell et al. 2005). The inclusion of Belemnitida and Diplobelida within the crown-group of the Decabrachia would simply solve this problem. Thus, the question if ancestral decabrachians had possessed a proostracum can be approved.
However, a gladius must not necessarily have been exposed from a proostracum-bearing phragmocone since experimental decalcification of a sepiid cuttlebone also exposes a gladius-like structure. By contrast to belemnoid coleoids, the sepiid lineage achieved a proostracum-like appearance through exponential growth of an endogastrically enrolled (proostracum-less) phragmocone (Haas 2003). This eccentric mode of growth, which can be observed in Cenozoic Ceratisepia and Belosaepia, caused the compression and later reduction of the ventral shell wall plus siphuncle (Fig. 3f). As a result of these phragmocone-related transformations (instead of only the terminal chamber wall), the sepiid cuttlebone moved from an originally posterior to a dorsal position and therefore appears proostracum-like. The derivation of a gladius through a proostracum-less cuttlebone is particularly attractive in topologies where Sepiida cluster with gladius-bearing Loliginida and Sepiolida, a grouping defined by the shared possession of a cornea and originally called “Myopsidae” by Orbigny, 1842 (=“Myopsida” sensu Haas 1997, 2003).
Independently if the latter argumentation proves correct or not, none of them would change the fact that at least some of the decabrachian gladius types underwent parallel modifications.
Bizikov (2008) included in his “loliginid type” gladiuses of loliginid (Loliginidae, Australiteuthidae) and bathyteuthoid families (Chtenopterygidae, Bathyteuthidae). Although Bathyteuthoidea are well-known to share characters with both Loliginida and Oegopsida, many analyses yielded a sister group relationship between oegopsids and bathyteuthoids (Strugnell and Nishiguchi 2007; Strugnell et al. 2005, 2006, 2009; Lindgren 2010; Allcock et al. 2011). If loliginids branched off from the sepioid line (Sepiida, Spirulida, Sepiolida), as many times suggested, the “similarity” in gladius characteristics between loliginids and bathyteuthoids must be considered as a parallelism. This appears surprising regarding identical muscular-gladius interactions and particularly with respect to ontogenetical features. The adult gladius of Chtenopteryx shows strong resemblance to paralarval gladiuses of loliginids and some oegopsids (Bizikov 2008). It seems as if loliginid and some oegopsid gladiuses pass through a “bathyteuthoid growth stage”, which would point to homologue mechanisms (and thus for the monophyly of Teuthoidea).
Ecological implications
“A predominant behavior (…) is such a strong selective force that homoplasy becomes a dominant source of the shared similarity…” (Hall 2007: p. 475).
Lindgren et al. (2012) has recognized various homoplasious traits (accessory nidamental glands, corneas, photophores, branchial canal, right oviduct) in the evolution of Recent Coleoidea and correlated them with adaptations to similar habitats and life styles. The same might be assumed for the iterative loss of calcification as well as for the parallel trends to develop similar gladius shapes.
The loss of calcification in different lineages is certainly linked with a shift from neutral to negative buoyancy. A new mode of buoyancy control could have either initiated adaptations to higher swimming velocities or to a more passive demersal life style. Recent oegopsids, bathyteuthoids, and loliginids have obviously perfected their jet-propulsion. The reduced state of their gladius, different life styles as well as their unsolved position within the phylogenetic tree make interpretations on the sepiolid and idiosepiid pathway difficult. Among Mesozoic gladius-bearing octobrachians, plesioteuthids, palaeololiginids, and muensterellids with their arrow-shaped, slender gladiuses were most probably the fastest invertebrate swimmers of the Mesozoic. However, the marginal attachment of the muscular mantle as well as the (putative) lack of mantle-locking cartilages was certainly less effective than in modern teuthoids (Fuchs et al. in press). The existence of “flying” octobrachians during the Mesozoic is therefore questionable. As can be judged from their proportionally wide and thick gladiuses, the loligosepiid and teudopseid branch was likely dominated by nectobenthic or benthic representatives.
Shared similarities in gladius shapes appears to be not or at least less driven by the life habitat. Owing to their biogeographical distributions, plesioteuthids, palaeololiginids, muensterellids and geopeltids were certainly inhabitants of shallower shelf habitats similar to living loliginids and unlike their Recent “counterparts”, which are adapted to deeper oceanic habitats.
Conclusions
An important event in the evolution of the coleoid locomotory system is certainly the invagination and hence internalization of the shell gland. However, the development of a distinctly forward projecting shell part, the proostracum, represents the seminal event responsible for the proliferation of the muscular mantle and thus the perfection of the jet-propulsion. The lamello-organic shell layer that mainly composes the proostracum is considered to be homologous to the main layer in fossil and Recent gladiuses. This means that a gladius has been embedded in every proostracum-bearing coleoid. The loss of calcification in any proostracum-bearing group accordingly exposes a gladius. Fossil and Recent gladiuses are in this light homologues with identical developmental mechanisms; similar gladius shapes in phylogenetically distant lineages are consequently parallelisms. A gladius can also be embedded in a proostracum-less phragmocone as the sepiid cuttlebone, which achieved a proostracum-like appearance through reduction of the ventral shell wall. Parallel gladius shapes can occur on superordinal (e.g. plesioteuthid Octobrachia—ommastrephid Decabrachia; palaeololiginid Octobrachia—bathyteuthoid Decabrachia) as well as on subordinal (plesioteuthid Prototeuthina—palaeololiginid Teudopseina) levels.
References
Allcock, L., Cooke, I., & Strugnell, J. (2011). What can the mitochondrial genome reveal about higher-level phylogeny of the molluscan class Cephalopoda? Zoological Journal of the Linnean Society, 161, 563–586.
Arkhipkin, A. I., Bizikov, V. A., & Fuchs, D. (2012). Vestigial phragmocone in the gladius points to a deep water origin of squid (Mollusca: Cephalopoda). Deep Sea Research: Oceanic Research Papers I, 61, 109–122.
Bandel, K., & Leich, H. (1986). Jurassic Vampyromorpha (dibranchiate cephalopods). Neues Jahrbuch für Geologie und Paläontologie Monatshefte, 1986(3), 129–148.
Barskov, I. S. (1973). Microstructure of the skeletal layers of Sepia and Spirula compared with the shell layers of other Molluscs. Paleontological Journal (Translation of the Paleontologicheskiy Zhurnal), 3, 285–294.
Berthold, T., & Engeser, T. (1987). Phylogenetic analysis and systematization of the Cephalopoda (Mollusca). Verhandlungen des Naturwissenschaftlichen Vereins Hamburg, 29, 187–220.
Bizikov, V. A. (1996). Atlas of morphology and anatomy of the gladius of squids. Moscow: VNIRO Publishing.
Bizikov, V. A. (2004). The shell in Vampyropoda (Cephalopoda): morphology, functional role and evolution. Ruthenica, supplement 3, 1–88.
Bizikov, V. A. (2008). Evolution of the shell in cephalopoda. Moscow: VNIRO Publishing.
Bizikov, V. A., & Toll, R. B. Part M, Chapter 9A: The gladius and its vestiges in Recent Coleoidea. Treatise Online (in press).
Boletzky, S. V. (1999). Brève mise au point sur la classification des céphalopodes actuels. Bulletin de Société Zoologique de France, 124(3), 271–278.
Carlini, D. B., Reece, K. S., & Graves, J. E. (2000). Actin gene family evolution and the phylogeny of coleoid Cephalopods. Molecular Biology and Evolution, 17(9), 1353–1370.
Clarke, M. R. (1988). Evolution of recent cephalopods—a brief review. In M. R. Clarke & E. R. Trueman (Eds.), The Mollusca. Paleontology and neontology (pp. 331–340). San Diego: Academic Press.
Doguzhaeva, L. A., Mapes, R. H., & Mutvei, H. (1999). A late carboniferous spirulid Coleoid from the southern mid-continent (USA). In F. Oloriz & F. J. Rodriguez-Tovar (Eds.), Advancing research on living and fossil cephalopds (pp. 47–57). New York: Kluwer Academic/Plenum Publisher.
Doguzhaeva, L. A., & Mutvei, H. (2003). Gladius composition and ultrastructure in extinct squid-like coleoids Loligosepia, Trachyteuthis and Teudopsis. Revue de Paléobiologie, 22(2), 877–894.
Doguzhaeva, L. A., & Mutvei, H. (2006). Ultrastructural and chemical comparison between gladiuses in living coleoids and Aptian coleoids from Central Russia. Acta Universitatis Carolinae—Geologica, 49, 83–93.
Doguzhaeva, L. A., Mutvei, H., & Weitschat, W. (2003). The Pro-ostracum and primordial rostrum at early Ontogeny of Jurassic Belemnites from North-Western Germany. Berliner Paläobiologische Abhandlungen, 3, 79–89.
Doguzhaeva, L. A., & Summesberger, H. (2012). Pro-ostraca of Triassic belemnoids (Cephalopoda) from Northern Calcareous Alps, with observations on their mode of preservation in an environment of northern Tethys which allowed for carbonization of non-biomineralized structures. Neues Jahrbuch für Geologie und Paläontologie—Abhandlungen, 266(1), 31–38.
Donovan, D. T. (1977). Evolution of the dibranchiate Cephalopoda. Symposia of the Zoological Society of London, 38, 15–48.
Donovan, D. T., Doguzhaeva, L. A., & Mutvei, H. (2003). Two pairs of fins in the Late Jurassic Coleoid Trachyteuthis from southern Germany. Berliner Paläobiologische Abhandlungen, 3, 91–99.
Donovan, D. T., & Fuchs, D. Part M, Chapter 10: Fossilized soft tissues in Coleoidea. Treatise Online (in press).
Donovan, D. T., & Strugnell, J. (2010). A redescription of the fossil coleoid cephalopod genus Palaeololigo Naef, 1921 and its relationship to recent squids. Journal of Natural History, 44(23–24), 1475–1492.
Doyle, P., Donovan, D. T., & Nixon, M. (1994). Phylogeny and systematics of the Coleoidea. The University of Kansas Paleontological Contributions (New Series), 5, 1–15.
Engeser, T., & Bandel, K. (1988). Phylogenetic classification of cephalopods. In J. Wiedmann & J. Kullman (Eds.), Cephalopods—present and past (pp. 105–115). Stuttgart: Schweizerbart’sche Verlagsbuchhandlung.
Fuchs, D. (2006a). Did early Decabrachia possess a proostracum in their body plan? Acta Universitatis Carolinae—Geologica, 49, 119–127.
Fuchs, D. (2006b). Diversity, taxonomy and morphology of vampyropod Coleoids (Cephalopoda) from the Upper Cretaceous of Lebanon. Memorie della Società Italiana di Scienze Naturali et del Museo Civico di Storia Naturale di Milano, 34(II), 1–28.
Fuchs, D. (2006c). Fossil erhaltungsfähige Merkmalskomplexe der Coleoidea (Cephalopoda) und ihre phylogenetische Bedeutung. Berliner Paläobiologische Abhandlungen, 8, 1–115.
Fuchs, D. (2009). Octobrachia—a diphyletic taxon? Berliner Paläobiologische Abhandlungen, 10, 182–192.
Fuchs, D., Boletzky, S. V., & Tischlinger, H. (2010). New evidence of functional suckers in belemnoid coleoids weakens support for the “Neocoleoidea” concept. Journal of Molluscan Studies, 76(4), 404–406.
Fuchs, D., Engeser, T., & Keupp, H. (2007a). Gladius shape variation in coleoid cephalopod Trachyteuthis from the Upper Jurassic Nusplingen and Solnhofen Plattenkalks. Acta Palaeontologica Polonica, 52(3), 575–589.
Fuchs, D., Iba, Y., Ifrim, C., Nishimura, T., Kennedy, J., Keupp, H., et al. (2013). Longibelus gen. nov., a new Cretaceous coleoid genus linking Belemnoidea and Decabrachia. Palaeontology, 56(5), 1081–1106.
Fuchs, D., Iba, Y., Tischlinger, H., Keupp, H., & Klug, C. On the locomotion system of fossil Coleoidea (Cephalopoda) and its phylogenetic significance. Lethaia (in press).
Fuchs, D., Keupp, H., Mitta, V., & Engeser, T. (2007b). Ultrastructural analyses on the conotheca of the genus Belemnotheutis (Belemnitida: Coleoidea). In R. H. Mapes & N. H. Landman (Eds.), Cephalopods present and past—new insights and fresh perspectives (pp. 300–315). Dortrecht: Springer.
Fuchs, D., Keupp, H., Trask, P., & Tanabe, K. (2012). Taxonomy, morphology and phylogeny of Late Cretaceous spirulid coleoids (Cephalopoda) from Greenland and Canada. Palaeontology, 55(2), 285–303.
Fuchs, D., Klinghammer, A., & Keupp, H. (2007c). Taxonomy, morphology and phylogeny of plesioteuthidid coleoids from the Upper Jurassic (Tithonian) Plattenkalks of Solnhofen. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 245(2), 239–252.
Fuchs, D., & Larson, N. L. (2011a). Diversity, morphology and phylogeny of coleoid cephalopods from the Upper Cretaceous Plattenkalks of Lebanon—part II: Teudopseina. Journal of Paleontology, 85(5), 815–834.
Fuchs, D., & Larson, N. L. (2011b). Diversity, morphology, and phylogeny of coleoid cephalopods from the Upper Cretaceous Plattenkalks of Lebanon—part I: Prototeuthidina. Journal of Paleontology, 85(2), 234–249.
Fuchs, D., & Weis, R. (2008). Taxonomy, morphology and phylogeny of Lower Jurassic loligosepiid coleoids (Cephalopoda). Neues Jahrbuch für Geologie und Paläontologie Abhandlungen, 249(1), 93–112.
Gray, J. E. (1849). Catalogue of the Mollusca in the collection of the British Museum. Part 1. Cephalopoda Antepedia. London: British Museum of Natural History.
Haas, W. (1997). Der Ablauf der Entwicklungsgeschichte der Decabrachia (Cephalopoda, Coleoidea). Paleontographica. Abt. A, 245, 63–81.
Haas, W. (2002). The evolutionary history of the eight-armed Coleoidea. In H. Summesberger, K. Histon, & A. Daurer (Eds.), Cephalopods—present and past (pp. 341–351). Wien: Abhandlungen der Geologischen Bundesanstalt.
Haas, W. (2003). Trends in the evolution of the Decabrachia. Berliner Paläobiologische Abhandlungen, 3, 113–129.
Hall, B. K. (2007). Homoplasy and homology: dichotomy or continuum? Journal of Human Evolution, 52, 473–479.
Jeletzky, J. A. (1966). Comparative morphology, phylogeny and classification of fossil Coleoidea. Paleontological Contributions, University of Kansas, Mollusca, 7, 1–166.
Khromov, D. N. (1990). Cuttlefishes in the systematics and phylogenie of the Cephalopoda. Zoologicheskiy Zhurnal, 69, 12–20.
Klug, C., Schweigert, G., Dietl, G., & Fuchs, D. (2005). Coleoid beaks from the Nusplingen Lithographic Limestone (Late Kimmeridgian, SW Germany). Lethaia, 38(3), 173–192.
Klug, C., Schweigert, G., Röper, M., Fuchs, D., & Tischlinger, H. New anatomical information on arms and fins from extraordinally preserved Plesioteuthis from the Late Jurassic of SE-Germany. Swiss Journal of Paleontology (in press).
Kröger, B., Vinther, J., & Fuchs, D. (2011). Cephalopod origin and evolution: a congruent picture emerging from fossils, development and molecules. BioEssays, 33(8), 602–613.
Lindgren, A. R. (2010). Molecular inference of phylogenetic relationships among Decapodiformes (Mollusca: Cephalopoda) with special focus on the squid Order Oegopsida. Molecular Phylogenetics and Evolution, 56(1), 77–90.
Lindgren, A. R., Giribet, G., & Nishiguchi, M. K. (2004). A combined approach to the phylogeny of Cephalopoda (Mollusca). Cladistics, 20, 454–486.
Lindgren, A., Pankey, M., Hochberg, F., & Oakley, T. (2012). A multi-gene phylogeny of Cephalopoda supports convergent morphological evolution in association with multiple habitat shifts in the marine environment. BMC Evolutionary Biology, 12(1), 129.
Naef, A. (1922). Die fossilen Tintenfische—Eine paläozoologische Monographie. Jena: Gustav Fischer.
Patterson, C. (1982). Morphological characters and homology. In K. A. Joysey & A. E. Friday (Eds.), Problems of phylogenetic reconstruction (pp. 21–74). London: Academic Press.
Remane, A. (1952). Die Grundlagen des natürlichen Systems der vergleichenden Anatomie und Phylogenetik. Leibzig: Geest und Portig K.G.
Riegraf, W., Janssen, N., Schmitt-Riegraf, C. (1998). Fossilium Catalogus. I: Animalia, Leiden: Backhys Publishers.
Roger, J. (1952). Sous-classes des Dibranchiata OWEN 1836. In J. Piveteau (Ed.), Traité de Paléontologie (pp. 689–755). Paris: Masson.
Strugnell, J., Jackson, J., Drummond, A. J., & Cooper, A. A. (2006). Divergence time estimates for major cephalopod groups: evidence from multiple genes. Cladistics, 22, 89–96.
Strugnell, J. M., Lindgren, A., & Allcock, A. L. (2009). Cephalopod mollusks (Cephalopoda). In S. B. Hedges & S. Kumar (Eds.), The tree of life (pp. 242–246). Oxford: University Press.
Strugnell, J., & Nishiguchi, M. K. (2007). Molecular phylogeny of coleoid cephalopods (Mollusca: Cephalopoda) inferred from three mitochondrial and six nuclear loci: a comparison of alignment, implied alignment and analysis methods. Journal of Molluscan Studies, 73, 399–410.
Strugnell, J., Norman, M., Jackson, J., Drummond, A. J., & Cooper, A. A. (2005). Molecular phylogeny of coleoid cephalopods (Mollusca: Cephalopoda) using a multiple approach; the effect of data partitioning on resolving phylogenies in a Bayesian framework. Molecular Phylogenetics and Evolution, 37, 426–441.
Toll, R. B. (1982). The Comparative Morphology of the Gladius in the Order Teuthoidea (Mollusca: Cephalopoda) in Relation to Systematics and Phylogeny. Miami: University of Miami.
Toll, R. B. (1988). Functional morphology and adaptive patterns of the teuthoid Gladius. In M. R. Clarke & E. R. Trueman (Eds.), The Mollusca. Form and function (pp. 167–182). New York: Academic Press.
Toll, R. B. (1998). The gladius in teuthid systematics. In: N. A. Voss, M. Vecchione, R. B. Toll, & M. J. Sweeney (Eds.), Systematics and Biogeography of Cephalopods (pp. 55–67), vol. II. 586 Smithsonian Contributions to zoology, Washington.
Young, R. E., & Vecchione, M. (1996). Analysis of morphology to determine primary sister-taxon relationships within coleoid cephalopods. American Malacological Bulletin, 12(1/2), 91–112.
Young, R. E., Vecchione, M., & Donovan, D. T. (1998). The Evolution of Cephalopods and their present Biodiversity and Ecology. South Africa Journal of Marine Science, 20, 393–420.
Acknowledgments
We are particularly grateful to Amanda Reid (Australian Museum, Sydney, Australia), Takenori Sakurai (University of Hokkaido, Hakodate, Japan) and Henk-Jan Hoving (GEOMAR, Kiel, Germany), who provided gladiuses of Neorossia, Todarodes, and Vampyroteuthis. Further thanks go to Alexander Arkhipkin (Falkland Islands Fishery Department) and two anonymous referees for their thorough proof reading. Finally, we compliment Christian Klug on the perfect organization of our meeting in Zurich. This research is funded by the JSPS Grant (No. 25800285) and MEXT grant for Tenure Tracking System (for Y. I).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuchs, D., Iba, Y. The gladiuses in coleoid cephalopods: homology, parallelism, or convergence?. Swiss J Palaeontol 134, 187–197 (2015). https://doi.org/10.1007/s13358-015-0100-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13358-015-0100-3