Abstract
Otophysi is one of the most important fish taxa of the world, as they make up for roughly 28% of all fish species and about two-thirds of all freshwater species worldwide. To understand their success and evolutionary history their sister-group, the Gonorynchiformes, take a key-position, e.g., for reconstructing morphological conditions in the latest common ancestors. Gonorynchiformes comprising only 40 species and have been often studied for that reason. Their pelvic girdle, however, got only little attention so far. Therefore, we studied this structure in extant gonorynchiforms and described the ontogeny in Kneria stappersii. In gonorynchiforms: (1) their basipterygium is principally flat (without dorsal or ventral projections) and placed in horizontal position, (2) has a ‘simple’ shape, i.e., it has only a single anterior process with small cartilaginous tips and becomes wider in its posterior part with a medial portion connecting to the basipterygium of the other side; (3) three radials and a pelvic splint are present, and (4) a prominent posterior process is missing. Although, the morphological situation is a lot similar as seen in clupeiforms and alepocephaliforms. Therefore, these characters have likely been present in the stem of Otomorpha, Ostariophysi, and Otophysi, but within the latter taxon eventually, a higher diversity of pelvic girdle morphology arose during evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the characters present in virtually all primary aquatic vertebrates is fins, which are essential for effective locomotion in a fluid environment. Besides unpaired dorsal, anal and caudal fins, gnathostomes possess two sets of paired fins: the pectoral and pelvic fins. Both got quite some attention in the context of the origin of land-living vertebrates, as the tetrapod limbs evolved from these paired fins (e.g., Shubin et al. 1997, 2006; Boisvert 2005; Clack 2009; Hogervorst et al. 2009; Schneider & Shubin 2013). Yet, among the more than 36.000 species of actinopterygians (Fricke et al. 2023) especially the pelvic girdle remains poorly studied. Only a few studies dealt with this structure in a broader taxonomic frame in actinopterygians (Stiassny & Moore 1992; Yamanoue et al. 2010; Keivany 2017). Some studies focused on polymorphism of the pelvic girdle within a certain taxonomic group to study trends in reduction during evolution or ecological adaptations, for example in ninespine stickleback Pungitius (Nelson 1971; Ziuganov & Zotin 1995), brook stickleback Culaea inconstans (Nelson & Atton 1971), threespine stickleback Gasterosteus aculeatus (Bell et al. 1993; Schröder et al. 2023), or Sander lucioperca (Ott et al. 2012). A few studies investigated the morphology of the pelvic girdle in selected taxonomic groups, like in phallosethids where this structure is transformed into a reproductive organ in males (Parenti 1986), or in liparids, cyclopterids, and gobies in which the pelvic fins are transformed into a sucking disk (Budney & Hall 2010). Further studies come from balitorids (Crawford et al. 2020), balistoids (Yamanoue et al. 2009), and ateleopodids (Atsumi 2016), Danio rerio (Grandel and Schulte-Merker 1998), or taxa with important phylogenetic position like Polypterus (Molnar et al. 2017) or sturgeons (Sewertzoff 1926). However, in many osteological studies of actinopterygians, the structures of the pelvic fin got only little attention. Hardly, any information on the pelvic girdle of extant Gonorynchiformes is given in the phylogenetic study of Grande & Poyato-Ariza (1999), and also not in a study summarizing their postcranial skeleton (Grande & Arratia 2010). Data from fossil relatives are rare, because the pelvic girdle is usually covered by other structures or in a more or less lateral position, where it is hard to identify individual structures. In rare occasions, the pelvic girdle is preserved in good condition, for example in †Mahengichthys singidaensis (Davis et al. 2013) which provides enough details for comparison.
Yet, gonorynchiforms comprise only 40 species (Fricke et al. 2023), but they are in an important phylogenetic position, as they present the sister-group of the Otophysi. Otophysi, on the other hand, are the world’s most diverse freshwater fish taxon (Arratia 2018; Betancur-R et al. 2017; Nakatani et al. 2011; Nelson et al. 2016; Rosen & Greenwood 1970). Therefore, to understand the evolutionary history of otophyseans, detailed studies of gonorynchiforms are needed for the reconstructions of character states at the base of Otophysi and Ostariophysi (i.e., Gonorynchiformes plus Otophysi). Besides descriptions of fossil gonorynchiforms, several aspects of the morphology of extant taxa were studied: Fink and Fink (1981, 1996) included gonorynchiforms in their seminal works on ostariophysean relationships; Rosen & Greenwood (1970) and Grande and de Pinna (2004) put some focus on the anterior-most vertebrae; Peters (1967) and Grande and Young (1997) investigated the opercular apparatus and the latter also the anterior vertebrae of Kneria; Johnson and Patterson (1997) described the gill arches of gonorynchiforms in detail; Grande and Poyato-Ariza (2010) reviewed the head skeleton; and Grande (1994) and Britz and Moritz (2007) studied the skeletal morphology of the miniaturized genera Cromeria and Grasseichthys. As only in the latter study, pelvic girdles are depicted in all details, information on the pelvic-girdle structures of Gonorynchiformes is still rare.
We were able to study all extant genera using cleared and double-stained specimens. Thereby, we are able to provide here a detailed study on the diversity of the pelvic girdle in the order Gonorynchiformes supplemented with ontogenetic data from Kneria stappersii.
Materials and methods
To examine the pelvic girdle, we used cleared and doubled-stained specimens. Clearing and staining for bone and cartilage principally followed the protocol of Dingerkus and Uhler (1977) and as described in Thieme et al. (2021) using Alcian blue for cartilage staining and Alizarin red for bone structures. The specimens were dissected and photographed using a Canon EOS 80D with a Sigma EX 105 mm objective. The pictures were than processed in Adobe Photoshop CC (version 22.0.5) to adjust the color values as well as Adobe Illustrator CC (version 25.2.3) to compile the plates. Specimens used in this study, including a developmental series of Kneria stappersii, are part of the ichthyological collection of the Deutsches Meeresmuseum. As clearing and double staining gives the best results in specimens below about 120 mm length, for adult Gonorynchus, a µCT-scan was used to check for possible discrepancies to the smaller cleared and stained specimen. Only large specimens of Chanos chanos, a species reaching well over 1 m in size, were not included in this study. Besides six genera of gonorynchiforms (Table 1) a set of comparative material from 38 species of alepocephaliforms, clupeiforms, and Otophysi has been investigated (Table 2). Information on the pelvic girdles of Cromeria nilotica and Grasseichthys gabonensis is taken from Britz and Moritz (2007).
Results
Comparative morphology of the pelvic girdle in Gonorynchiformes
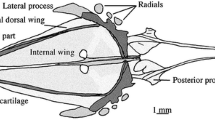
Principally, the morphology of the pelvic girdle in gonorynchiforms is very similar (Fig. 1). The basipterygium is roughly rod-shaped to triangular and can be divided into an anterior process and a posterior portion comprising a medial arm and the articulatory margin for the radials and fin rays. It is flat, bare of prominent dorsal or ventral projections, and embedded close under the skin in the body, without any direct connection to the remaining skeleton. In principle, in gonorynchiforms the pelvic fin is positioned roughly below the dorsal fin. In detail, there are slight differences between taxa, e.g., in Kneria and Cromeria occidentalis, it starts slightly anterior, and clearly anterior in Cromeria nilotica. In Phractolaemus, the pelvic fin even ends in front of the beginning of the dorsal fin. The medial arm at the posterior end approaches the medial arm of the other side and is loosely, e.g., in Chanos (Fig. 1c), or tightly, e.g., Kneria (Fig. 1a) and Phractolaemus (Fig. 1b) connected via cartilaginous tissue. Juvenile Gonorynchus do not yet have the posterior medial portions of the basipterygia connected to each other (Fig. 1d), but in larger specimens, e.g., our µCT-scanned specimen, medial arms approaching each other are visible. In Phractolaemus and Chanos (Fig. 1b, c), the medial arm is relatively short. In Kneria, Parakneria, and Cromeria occidentalis, the medial arm is longer and more pronounced than in the other taxa. While it is straight in Kneria and Parakneria, the medial arm in Cromeria occidentalis is slightly distally bent. A posterior process as known from many teleosts is rather small or absent in gonorynchiforms. It is most expressed in Chanos, yet is does not surpass the tip of the medial radial posteriorly in our specimen. Posterior processes as tiny tips are present in Kneria and Phractolaemus. In Parakneria sp. ‘mukuleshi’, the situation is more complicated: there is a very tiny tip at the position where Kneria has its posterior process, but slightly before that there is another posterior directed outgrowth, a secondary posterior process. It is flat and bifurcated. In C. occidentalis, the posterior process is absent and the amount of cartilage at the posterior part of the pelvic girdle is generally higher than in the other genera studied. The posterior process is also missing in the studied cleared and double-stained specimen of G. abbreviatus, but a small posterior process is visible in an adult individual (µCT-Scan).
Diversity of the pelvic fin in Gonorynchiformes in ventral view. a Kneria stappersii (IE/12025, SL = 25.1 mm). b Phractolaemus ansorgii (IE/11045, SL = 49.0 mm). c Chanos chanos (IE/11010, SL = 66.0 mm). d Gonorynchus abbreviatus (IE/16935, SL = 78.2 mm). as axillary scale, bspt basipterygium, fr fin ray, lw lateral wing, ma medial arm, mw medial wing, pp posterior process, ps pelvic splint, r radial
The tips of the anterior processes are cartilaginous and draw near to each other, with the exception of Phractolaemus where the anterior sections of the anterior processes are almost in parallel position, so that the tips do not touch (Fig. 1). Along the anterior process, there is medially a thin membrane ossification, which is pronounced more or less in the different taxa. In Phractolaemus, this ‘wing’ is relatively small; additionally, there is also a lateral wing, which is more pronounced than the medial one and unique within gonorynchiforms (Fig. 1b). The medial wing is positioned in a medial slightly ventral direction. The posterior margin of the basipterygium is generally covered with cartilage. Directly on this margin, there are usually three radials (called ‘pterygophore’ by Thys van den Audenaerde 1961). Two out of 22 studied individuals of C. occidentalis had two radials on one side of the body, but in general, there are three. The lateral and middle radials are in most cases to some extent rounded (Fig. 1a, c). In Gonorynchus, the middle radial is elongated (Fig. 1d), and in Phractolaemus, the most lateral radial is elongated (Fig. 1b).
The number of fin rays in the pelvic fin ranges from six in Phractolaemus and C. occidentalis to ten in Chanos (Table 3). An axillary scale is present only in Gonorynchus and Chanos (Fig. 1c, d). In both species, small typical body scales cover the base of this structure. The axillary scales are delicate and elongated structures, which are located laterally of the pelvic fin. In Gonorynchus, there is an elongated core visible, which stains blue and slightly red in cleared and double-stained specimens. The axillary scale in Chanos is broader and the inner supporting structure consists of multiple semi-circular parts in a row, which also stain blue in cleared and double-stained specimens. A pelvic splint is present in all examined species. It is located on the lateral margin of the pelvic fin and does not articulate with any radial. In P. ansorgii and C. chanos, it is located very close to the first fin ray, but in the other taxa, there can be a more or less expressed gap between splint and ray (Fig. 1). It has a delicate rod-shape with pointed ends and looks similar to an ‘L’. Usually, the shorter side is directed toward the inside of the body. Only in P. ansorgii, the pelvic splint is slightly broader and the proximal part is longer than the distal one.
Development of the pelvic girdle in Kneria stappersii
The development of the pelvic fin starts at about 10 mm body length with the formation of paired fin lobes at the position of the future pelvic fin. In these lobes, densely set actinotrichia are visible. The basipterygia become visible as two, simple rod-shaped cartilages at around 10.2 mm (Fig. 2a). Although slightly smaller, a specimen of 9.5 mm SL nicely illustrated a following stage: shortly after the first three ossified fin rays are visible and the basipterygium widens at the posterior end where the fin rays are based (Fig. 2b). In a specimen of 13.4 mm, all elements of the pelvic girdle are present: the basipterygium, three radials, eight fin rays, and the pelvic splint at the lateral side of the pelvic girdle are also visible but not fully developed (Fig. 2c). The posterior end of the basipterygia has broadened even more, the medial arms of both sides are connected through a continuous bridge of cartilage cells, and only the bases of both medial arms are ossified. At this juvenile stage, the ‘wing’-like medial membrane of the basipterygium is already partly developed (Figs.1a, 2c), has no cartilaginous precursor, and thus develops through membrane ossification. Of the three radials, the two smaller ones are still cartilaginous, while the bigger, most medial one is already ossified. The pelvic splint is still in its early development. Its proximal portion is smaller than in later stages. In contrast to the already complete and fully developed fin rays, it appears quite delicate. During further growth, the medial arms continue to ossify and all radials become fully ossified. Furthermore, the pelvic splint gets a more pronounced ‘L’ shape, with the shorter arm pointing toward the body.
Discussion
Diversity in Gonorynchiformes
So far, little attention was given to the pelvic girdles of gonorynchiforms. The position of the insertion of the pelvic fin relative to the origin of the dorsal fin was used as one of several characters to distinguish the genera Parakneria and Kneria: in the first, the dorsal fin origin is in front of the pelvic-fin origin, while in Kneria, the pelvic fin lies in more anterior position (Poll 1965). In contrast to the statement of Giltay (1934) that in Kneria auriculata, the ventral fin is directly below the origin of the dorsal fin, and the statement of Grande and Arratia (2010:56) that in gonorynchiforms, ‘the dorsal fin is always anterior to the pelvic fins’, this character is in general valid. As Mutambala et al. (2022) recently reviewed this issue, only single specimens may deviate from the genus specific position of the pelvic fin.
Position, shape, and number of elements are similar within Gonorynchiformes (Table 3); nevertheless, all taxa show some peculiarities. The number of fin rays is not much different with 10 in the largest representative Chanos and only 6 and 7 in Phractolaemus, Cromeria nilotica, and Grasseichthys. Phractolaemus is unique in having a lateral wing and Parakneria in having a secondary posterior process. Our cleared and stained Gonorynchus specimen is virtually missing the medial arm, the contact of both basipterygia in this area and a posterior process. Nevertheless, it must be taken into account that the studied individuals were relatively young and small, and accordingly, these structures seem to grow during further development. Grande and Poyato–Ariza (1999) described without giving an illustration a ‘fleshy lobe’ and a ‘cartilaginous filament or fin ray’ in the pelvic girdle of Gonorynchus. The authors likely described what we regard as the axillary scale, which is in larger specimens covered with small regular scales. In the cleared and stained specimen, the axillary reveals a central, delicate rod-like supporting structure. This structure stains blue, but apparently due to strong connective tissue, as we did not observe cartilage cells. Following our interpretation, the ‘fleshy lobe’ of Grande and Poyato-Ariza (1999) is not unique for Gonorynchus, but represents an axillary scale, which is also present in Chanos (Fig. 1c). Chanos is regarded as sister-group to the other Gonorynchiformes (Rosen and Greenwood 1970; Fink and Fink 1981; Poyato–Ariza et al. 2010). A comparison with their close relatives within the Otomorpha shows that the pelvic-girdle situation found in Chanos seems to be close to the characteristics of the last common ancestor of Gonorynchiformes. The poorly developed medial arms in Gonorynchus is probably a derived reductions and no primitive state. The conditions found in Kneriidae is similar to their fossil relative †Mahengichthys singidaensis (Davis et al. 2013). The rod-shaped basipterygium is on the lateral side and a thin wing-like structure on the medial side of the pelvic girdle. The number of fin rays, eight per side, is perfectly in range of kneriids. Also, a small pelvic splint on the lateral side of the fin rays and a small median arm of the basipterygium are preserved. Most related fossil taxa show only incomplete preservation of the pelvic girdle. The stem-group gonorynchiform †Tischlingerichthys viohli has a rather triangular basipterygium probably resulting from a much pronounced lateral wing (Arratia 1997). Also, a splint is present, but for probably cartilaginous structures like posterior process or total amount of radials, no information is available.
The effect of miniaturization in the gonorynchiform pelvic girdle
Some attention was laid on the miniaturization in Gonorynchiformes (Grande 1994; Britz and Moritz 2007; Lavoué et al. 2012). Due to their elongated body shape, both Cromeria species surpass the definition of miniature fish given by Weitzman and Vari (1988), i.e., becoming sexually mature under 20 mm or not exceeding 26 mm standard length. Nevertheless, most skeletal elements like skull and caudal fin are in the size of typically miniature fish species and Cromeria as Grasseichthys show many morphological characters typical for miniaturization (Britz & Moritz 2007). Such characteristics for miniaturization are (1) increased morphological variability, (2) reduction and structural simplification, (3) hyperossification, and (4) morphological novelty (Hanken and Wake 1993; Britz and Moritz 2007). Solely regarding the pelvic girdle of Grasseichthys and Cromeria, these characteristics, however, are poorly expressed. Cromeria occidentalis illustrates that even in an adult specimen, the posterior part of the basipterygium is still mostly cartilaginous and less ossified than in other gonorynchiforms (Britz and Moritz 2007: Fig. 11). There is only a limited morphological variability in the number of radialia: for the present study, 22 cleared and double-stained C. occidentalis were examined of which two specimens had only two radialia in one of the sides. Although pelvic-fin reduction and even complete loss happened several times during teleost evolution (Yamanoue et al. 2010), miniaturization is not a driver for pelvic-fin loss, which can also be seen by well-developed pelvic girdles in other miniaturized ostariophyseans, e.g., Priocharax (Mattox et al. 2016), Paedocypris (Britz & Conway 2009), or Micromyzon (Carvalho et al. 2016).
The ontogeny of the pelvic girdle
The ontogenetic development of the pelvic girdle in Kneria stappersii resembles much to the development in Chanos chanos (Taki et al. 1986; Arratia and Bagarinao 2010). The ontogenetic development, in both species, starts with a rod-shaped cartilaginous precursor of the basipterygium, which soon broadens at the posterior portion. The next element to be visible were some of the fin rays as direct ossification and shortly after the radials in cartilage. The third and largest radial developed with a time lag from the others. While Taki et al. (1986) reported in total only three radials, as we found in our specimen, Arratia & Bagarinao (2010) showed that the most lateral cartilage during further growths separates in two pieces, finally resulting in four radials in Chanos. Formation of the fin rays apparently starts with the first (lateral) ray and the medial rays follow in row. Only the splint appears significantly later (Arratia and Bagarinao 2010). While in Chanos, ossification of the basipterygium starts at the same time when the first ray appears (Arratia & Bagarinao 2010), the ossification of the basipterygium in Kneria happens slightly later. One difference in the development is a foramen that is formed in the posterior region of the basipterygium in Chanos (Taki et al. 1986), but is absent in K. stappersii.
Comparison with Ostariophysi
To place the obtained information about the pelvic girdle in Gonorynchiformes in a phylogenetic context, 38 species from different orders within the non-euteleost teleosts (NETs) were additionally studied (Table 3). At the base of the Otomorpha are the Clupeiformes and Alepocephaliformes (Arratia 2018; Bentacur-R et al. 2017; Straube et al. 2018) and both taxa share some characters in the pelvic girdle with the Gonorynchiformes. The basipterygium of all three orders has only a single anterior process, is similar rod- to triangular-shaped without dorsal or ventral flanges or outgrowths, and positioned parallel to the ventral body side. In clupeids, there is no or no prominent posterior process found like in gonorynchiforms. Clupeids are missing the pelvic splint (Fig. 3b), which is present in alepocephaliforms and gonorynchiforms, as well as in most other NETs. The skeleton of the deep-sea dwelling Alepocephaliformes has a higher proportion of cartilage (Fig. 3a), which can also be seen in the pelvic girdle, where a significant posterior portion of the basipterygium is largely cartilaginous. Within Ostariophysi, the pelvic girdle of characiforms and cypriniforms has many similarities (Fig. 3c, d). Gymnotiformes have reduced their pelvic girdle (Fink and Fink 1981, 1996), and in Siluriformes, we find a high diversity (e.g., de Pinna 1993; Shelden 1937; Silva Pedroza 2009) which will be focused on in a forthcoming study. The phylogenetic interrelationships of Characiformes are still unclear to date also on their base. Morphological studies (e.g., Fink and Fink 1981, 1996) and some recent molecular investigations (e.g., Bentacur-R et al. 2017) regard characiforms as monophyletic. Yet, many molecular studies put doubt in this by supporting two separate taxa, the Citharinoidei and Characoidei (e.g., Ortí & Meyer 1996, 1997; Nakatani et al. 2011; Chen et al. 2013; Arcila et al. 2017; Hughes et al. 2018). The question of the possible paraphyly of characiforms will have remarkable impact in future studies on the interpretation of character states in Otophysi, which so far consisted of only four major taxa. Concerning the pelvic girdle, this impact remains, however, limited. The basipterygia in Distichodontidae have a bifurcation, i.e., there are two anteriorly directed processes. The situation in the other family of the Citharinoidei, the Citharinidae, needs further detailed studies: while Vari (1979: 311) confirms a bifurcated basipterygium for citharinids, Fink and Fink (1996: 240) contradict this statement. Also, our specimen of Citharinus eburneensis does not have an anteriorly bifurcated basipterygium. For a final statement of the situation in citharinids, more taxa need to be studied in detail. In the Characoidei, there is no bifurcation of the basipterygium visible at least in adults. In some species, e.g., Chilodus punctatus, a second more dense structure in the ‘wing’-like medin area of the basipterygium is apparent, which could be a remainder of a second anterior process; ontogenetic studies may solve this issue. In the ontogeny of some characiforms like Hepsetus, a bifurcated basipterygium has been reported from early stages (Fink and Fink 1981).
Pelvic girdle of different otomorph species, ventral view. a Alepocephalus bicolor (IE/13719; SL = 109.0 mm). b Clupea harengus (IE/17219; SL = 83.2 mm). c Phoxinus phoxinus (IE/16931; SL = 50.1 mm). d Ichthyborus besse (IE/11807; SL = 46.2 mm). as axillary scale, bspt basipterygium, fr fin ray, ma medial arm, map medial posterior process, pp posterior process, ps pelvic splint, r radial
Cypriniformes generally possess a bifurcated basipterygium (Fig. 3c). Cleared and stained specimens show that the lateral anterior process in cypriniforms and characiforms forms enchondral, as there remains a cartilaginous tip throughout life.
Otophysi usually have well-developed posterior processes, even if there is a lot of variability in shape and size (e.g., Takeuchi and Hosoya 2011; Nasri et al. 2013; Azimi et al. 2015). In gonorynchiforms, it is only little developed, so that a marked posterior process originated within the stem group of Otophysi. Well-developed posterior processes in various euteleost taxa, e.g., Myctophiformes, Stomiiformes, Salmoniformes, Zeiformes, Aulopiformes, and Lepidogalaxiias, therefore have convergently developed. Also, the evolution of a second anterior process happened within the otophysean stem group. A pelvic splint is present in Gonorynchiformes, Alepocephaliformes, as well as most Characiformes and Cypriniformes and many Siluriformes. In all NETs, the pelvic girdle is positioned relatively flat under the skin on the ventral side of the body. Including the results from Alepocephaliformes and Clupeiformes, it seems that already in the last common ancestor of Otomorpha, there is a rod- to triangular-shaped basipterygium with three radials and a pelvic splint; a posterior process is only little expressed. The very broad, triangular basipterygium of the stem group gonorynchiform †Tischlingerichthys viohli (Arratia 1997) is likely a derived character. Summarizing this comparison confirms the statement of Fink and Fink (1981, 1996) that the primitive condition for the pelvic girdle of Gonorynchiformes seems virtually identical with the possible situation in the stem of Ostariophysi.
Data availability
All the cleared and stained specimen used for this study are deposited and accessible at the Deutsches Meeresmuseum.
References
Arcila D, Ortí G, Vari R et al (2017) Genome-wide interrogation advances resolution of recalcitrant groups in the tree of life. Nature Ecol Evol. https://doi.org/10.1038/s41559-016-0020
Arratia G (1997) Basal teleosts and teleostean phylogeny. Dr. Friedrich Pfeil, Palaeo Ichthyologica, München. https://doi.org/10.2307/1447369
Arratia G (2018) Otomorphs (= otocephalans or ostarioclupeomorphs) revisited. Neotropical Ichthyology 16(3):e180079. https://doi.org/10.1590/1982-0224-20180079
Arratia G, Bagarinao T (2010) Early ossification and development of the cranium and paired girdles of Chanos chanos (Teleostei, Gonorynchiformes). Gonorynchiformes and ostariophysan relationships: a comprehensive review. Science Publishers, Enfield, pp 73–106
Atsumi K (2016) Anatomical features of the pelvic girdle in the family Ateleopodidae (Pisces: Ateleopodiformes). Bull Fish Sci Hokkaido Univ 66(2):59–61. https://doi.org/10.14943/bull.fish.66.2.59
Azimi H, Mousavi-Sabet H, Eagderi S (2015) Osteological characteristics of Paraschistura nielseni (Nalbant & Bianco, 1998) (Cypriniformes: Nemacheilidae). Iran. J. Ichthyol. 2(3):155–164. https://doi.org/10.22034/iji.v2i3.61
Bell MA, Ortí G, Walker JA, Koenings JP (1993) Evolution of pelvic reduction in threespine stickleback fish: a test of competing hypotheses. Evolution 47(3):906–914. https://doi.org/10.1111/j.1558-5646.1993.tb01243.x
Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Orti G (2017) Phylogenetic classification of bony fishes. BMC Evol Biol 17(1):1–40. https://doi.org/10.1186/s12862-017-0958-3
Boisvert CA (2005) The pelvic fin and girdle of Panderichthys and the origin of tetrapod locomotion. Nature 438:1145–1147. https://doi.org/10.1038/nature04119
Britz R, Conway KW (2009) Osteology of Paedocypris, a miniature and highly developmentally truncated fish (Teleostei: Ostariophysi: Cyprinidae). J Morph 270(4):389–412. https://doi.org/10.1002/jmor.10698
Britz R, Moritz T (2007) Reinvestigation of the osteology of the miniature African freshwater fishes Cromeria and Grasseichthys (Teleostei, Gonorynchiformes, Kneriidae), with comments on kneriid relationships. Mitt Mus Naturkunde Berl, Zoolog Reihe 83(1):3–42. https://doi.org/10.1002/mmnz.200600016
Budney L, Hall B (2010) Comparative morphology and osteology of pelvic fin-derived midline suckers in lumpfishes, snailfishes and gobies. J App Ichthyol 26(2):167–175. https://doi.org/10.1111/j.1439-0426.2010.01398.x
Carvalho TP, Lundberg JG, Baskin JN, Friel JP, Reis RE (2016) A new species of the blind and miniature genus Micromyzon Friel and Lundberg, 1996 (Silurifomes: Aspredinidae) from the Orinoco River: describing catfish diversity using high-resolution computed tomography. Proc Acad Natl Sci Phila 165(1):37–53. https://doi.org/10.1635/053.165.0104
Chen W-J, Lavoué S, Mayden RL (2013) Evolutionary origin and early biogeography of otophysan fishes (Ostariophysi: Teleostei). Evolution 67(8):2218–2239. https://doi.org/10.5061/dryad.62t87
Clack JA (2009) The fin to limb transition: new data, interpretations, and hypotheses from paleontology and developmental biology. Annu Rev Earth Planet Sci 37:163–179. https://doi.org/10.1146/annurev.earth.36.031207.124146
Crawford CH, Randall ZS, Hart PB, Page LM, Chakrabarty P, Suvarnaraksha A, Flammang BE (2020) Skeletal and muscular pelvic morphology of hillstream loaches (Cypriniformes: Balitoridae). J Morph 281(10):1280–1295. https://doi.org/10.1002/jmor.21247
Davis MP, Arratia G, Kaiser TM (2013) The first fossil shellear and its implications for the evolution and divergence of the Kneriidae (Teleostei: Gonorynchiformes). In: Arratia G, Schultze HP, Wilson MVH (eds.), Mesozoic Fishes 5—Global Diversity and Evolution. Dr. Pfeil Verlag, München, pp 325–362
Dingerkus G, Uhler LD (1977) Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol 52(4):229–232. https://doi.org/10.3109/10520297709116780
Fink SV, Fink WL (1981) Interrelationships of the ostariophysan fishes (Teleostei). Zool J Linn Soc 72:297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
Fink SV, Fink WL (1996) Interrelationships of ostariophysan fishes (Teleostei). In: Stiassny MLJ, Parenti LR, Johnson GD (eds) Interrelationships of fishes. Academic Press, San Diego, pp 209–249
Fricke R, Eschmeyer WN, Fong JD (2023) Eschmeyer’s catalog of fishes: genera/species by family/subfamily. Accessed 15 Aug 2023. http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
Giltay L (1934) Contribution à l’étude du genre Xenopomatichthys (Kneriidae). Bull Du Musée Royal D’histoire Naturelle De Belgique Tome X, No 44:1–22
Grande T (1994) Phylogeny and paedomorphosis in an African family of freshwater fishes (Gonorynchiformes: Kneriidae). Fieldiana 78:1–20
Grande T, Arratia G (2010) Morphological analysis of the gonorynchiform postcranial skeleton. In: Grande T, Poyato-Ariza FJ, Diogo R (eds) Gonorynchiformes and ostariophysan relationships—a comprehensive review. Science Publishers, Enfield, pp 39–71
Grande T, de Pinna M (2004) The evolution of the Weberian apparatus: a phylogenetic perspective. In: Arratia G, Tintori A (eds) Mesozoic Fishes 3—systematics, paleoenvironments and biodiversity. Verlag Dr, Friedrich Pfeil, Munich, pp 429–448
Grande T, Poyato-Ariza FJ (1999) Phylogenetic relationships of fossil and recent gonorynchiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc 125:197–238. https://doi.org/10.1111/j.1096-3642.1999.tb00591.x
Grande T, Poyato-Ariza FJ (2010) Reassessment and comparative morphology of the gonorynchiform head skeleton. In: Grande T, Poyato-Ariza FJ, Diogo R (ets) Gonorynchiformes and Ostariophysan Relationships – A Comprehensive Review. Science Publishers, Enfield, pp 1–37
Grande T, Young B (1997) Morphological development of the opercular apparatus in Kneria wittei (Ostariophysi: Gonorynchiformes) with comments on its possible function. Acta Zool 78(2):145–162. https://doi.org/10.1111/j.1463-6395.1997.tb01134.x
Grandel H, Schulte-Merker S (1998) The development of the paired fins in the zebrafish (Danio rerio). Mech Dev 79(1–2):99–120. https://doi.org/10.1016/S0925-4773(98)00176-2
Hanken J, Wake DB (1993) Miniaturization of body size: organismal consequences and evolutionary significance. Annu Rev Ecol Syst 24:501–519
Hogervorst T, Bouma HW, De Vos J (2009) Evolution of the hip and pelvis. Acta Orthop 80(sup336):1–39. https://doi.org/10.1080/17453690610046620
Hughes LC, Ortí G, Huang Y et al (2018) Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Biological Sci 115(24):6249–6254. https://doi.org/10.1073/pnas.1719358115
Johnson GD, Patterson C (1997) The gill-arches of gonorynchiform fishes. S Afr J Sci 93:594–600
Keivany Y (2017) Osteological features of eurypterygian pelvic girdles. Iran J Sci Technol, Trans a: Sci 41(4):989–1002. https://doi.org/10.1007/s40995-017-0324-8
Kiwele Mutambala P, Abwe E, Schedel FDB, Manda AC, Schliewen UK, Vreven EJWMN (2022) A new Parakneria Poll 1965 (Gonorynchiformes: Kneriidae), ‘Mikinkidi’ from the upper Lufira Basin (Upper Congo: DCR): evidence from a morphologic and DNA barcoding integrative approach. J Fish Biol. https://doi.org/10.1111/jfb.15206
Lavoué S, Miya M, Moritz T, Nishida M (2012) A molecular timescale for the evolution of the African freshwater fish family Kneriidae (Teleostei: Gonorynchiformes). Ichthyol Res 59:104–112. https://doi.org/10.1007/s10228-011-0258-7
Lenglet G (1974) Contribution a l’étude ostéologique des Kneriidae. Ann Soc R Zool Bel 22:52–103
Mattox GMT, Britz R, Toledo-Piza M (2016) Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J Morph 277(1):65–85. https://doi.org/10.1002/jmor.20477
Molnar JL, Johnston PS, Esteve-Altava B, Diogo R (2017) Musculoskeletal anatomy of the pelvic fin of Polypterus: implications for phylogenetic distribution and homology of pre-and postaxial pelvic appendicular muscles. J Anat 230(4):532–541. https://doi.org/10.1111/joa.12573
Moritz T, Britz R, Linsenmair KE (2006) Cromeria nilotica and C. occidentalis, two valid species of the African freshwater fish family Kneriidae (Teleostei: Gonorynchiformes). Ichthyol. Explor. Freshwater 17(1):65–72
Nakatani M, Miya M, Mabuchi K, Saitoh K, Nishida M (2011) Evolutionary history of Otophysi (Teleostei), a major clade of the modern freshwater fishes: Pangaean origin and Mesozoic radiation. BMC Evol Biol 11(1):1–25. https://doi.org/10.1186/1471-2148-11-177
Nasri M, Keivany Y, Dorafshan S (2013) Comparative osteology of lotaks, Cyprinion kais and C. macrostomum (Cypriniformes, Cyprinidae), from Godarkhosh River, Western Iran. J Ichthyol 53(6):455–463. https://doi.org/10.1134/S0032945213040103
Nelson JS (1971) Absence of the pelvic complex in ninespine sticklebacks, Pungitius pungitius, collected in Ireland and Wood Buffalo National Park Region, Canada, with notes on meristic variation. Copeia 1971: 707-717. https://doi.org/10.2307/1442641
Nelson JS, Atton FM (1971) Geographic and morphological variation in the presence and absence of the pelvic skeleton in the brook stickleback, Culaea inconstans (Kirtland). Alberta and Saskatchewan Can J Zool 49(3):343–352. https://doi.org/10.1139/z71-050
Nelson JS, Grande TC, Wilson MVH (2016) Fishes of the World. John Wiley & Sons, Hoboken
Ortí G, Meyer A (1996) Molecular evolution of ependymin and the phylogenetic resolution of early divergences among euteleost fishes. Mol Biol Evol 13(4):556–573.
Ortí G, Meyer A (1997) The radiation of characiform fishes and the limits of resolution of mitochondrial ribosomal DNA sequences. Syst Biol 46(1):75–100. https://doi.org/10.2307/2413637
Ott A, Löffler J, Ahnelt H, Keckeis H (2012) Early development of the postcranial skeleton of the pikeperch Sander lucioperca (Teleostei: Percidae) relating to developmental stages and growth. J Morph 273:894–908. https://doi.org/10.1002/jmor.20029
Parenti LR (1986) Homology of pelvic fin structures in female phallostethid fishes (Atherinomorpha, Phallostethidae). Copeia 1986: 305-310. https://doi.org/10.2307/1444991
Peters N (1967) Opercular- und Postopercularorgan (Occipitalorgan) der Gattung Kneria (Kneriidae, Pisces) und ein Vergleich mit verwandten Strukturen. Z Morph Ökol Tiere 59(4):381–435. https://doi.org/10.1007/BF00409149
de Pinna MCC (1993) Higher-level phylogeny of Siluriformes, with a new classification of the order (Teleostei, Ostariophysi). Dissertation, City University of New York
Poll M (1965) Contribution à l’étude des Kneriidae et description d’un nouveau genre, le genre Parakneria (Pisces, Kneriidae). Mem Acad R Belg Cl Sci 36(4):1–28
Poyato-Ariza FJ, Grande T, Diogo R (2010) Gonorynchiform interrelationships: historic overview, analysis, and revised systematics of the group. In: Grande T, Poyato-Ariza FJ, Diogo R (eds) Gonorynchiformes and ostariophysan relationships—a comprehensive review. Science Publishers, Enfield, pp 227–338
Rosen DE, Greenwood PH (1970) Origin of the Weberian apparatus and the relationships of the ostariophysan and gonorynchiform fishes. Am Mus Novit 2428:1–25
Schneider I, Shubin NH (2013) The origin of the tetrapod limb: from expeditions to enhancers. Trends Genet 29(7):419–426. https://doi.org/10.1016/j.tig.2013.01.012
Schröder M, Windhager S, Schaefer K, Ahnelt H (2023) Adaptability of bony armor elements of the threespine stickleback Gasterosteus aculeatus (Teleostei: Gasterosteidae): ecological and evolutionary insights from symmetry analyses. Symmetry 15(4):811. https://doi.org/10.3390/sym15040811
Sewertzoff A (1926) Development of the pelvic fins of Acipenser ruthenus. New data for the theory of the paired fins of fishes. J Morph 41(2):547–579. https://doi.org/10.1002/jmor.1050410208
Shelden FF (1937) Osteology, Myology, and probable evolution of the nematognath pelvic girdle. Ann N Y Acad Sci 37(1):1–95. https://doi.org/10.1111/j.1749-6632.1937.tb55481.x
Shubin NH, Tabin C, Carroll S (1997) Fossils, genes and the evolution of animal limbs. Nature 388:639–648. https://doi.org/10.1038/41710
Shubin NH, Daeschler EB, Jenkins FA (2006) The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature 440:764–771. https://doi.org/10.1038/nature04637
Silva Pedroza WS (2009) Morfologia comparada da cintura pélvica de representantes da superfamília loricarioidea (ostariophysi siluriformes). Dissertation, INPA/UFAM, Manaus, Brazil
Stiassny ML, Moore JA (1992) A review of the pelvic girdle of acanthomorph fishes, with comments on hypotheses of acanthomorph intrarelationships. Zool J Linn Soc 104:209–242. https://doi.org/10.1111/j.1096-3642.1992.tb00923.x
Straube N, Li C, Mertzen M, Yuan H, Moritz T (2018) A phylogenomic approach to reconstruct interrelationships of main clupeocephalan lineages with a critical discussion of morphological apomorphies. BMC Evol Biol 18:1–17. https://doi.org/10.1186/s12862-018-1267-1
Takeuchi H, Hosoya K (2011) Osteology of Ischikauia steenackeri (Teleostei: Cypriniformes) with comments on its systematic position. Ichthyol Res 58:10–18. https://doi.org/10.1007/s10228-010-0181-3
Taki Y, Kohno H, Hara S (1986) Early development of fin-supports and fin-rays in the milkfish Chanos chanos. Jpn J Ichthyol 32(4):413–420
Thieme P, Warth P, Moritz T (2021) Development of the caudal-fin skeleton reveals multiple convergent fusions within Atherinomorpha. Front Zool 18:1–19. https://doi.org/10.1186/s12983-021-00408-x
Thys van den Audenaerde DFE (1961) L’anatomie de Phractolaemus ansorgei Blgr et la position systématique des Phractolaemidae. Ann Mus Roy Afr Centr, Sci Zool Sér 8(103):101–167
Vari RP (1979) Anatomy, relationships and classification of the families Citharinidae and Distichodontidae (Pisces, Characoidea). Bull Brit Mus (Natur Hist). Zool 36:261–344. https://doi.org/10.5962/bhl.part.3608
Weitzman SH, Vari RP (1988) Miniaturization in south american freshwater fishes; an overview and discussion. Proc Biol Soc Wash 101(2):444–465
Yamanoue Y, Miya M, Matsuura K, Sakai H, Katoh M, Nishida M (2009) Unique patterns of pelvic fin evolution: a case study of balistoid fishes (Pisces: Tetraodontiformes) based on whole mitochondrial genome sequences. Mol Phylogenetics Evol 50(1):179–189. https://doi.org/10.1016/j.ympev.2008.10.016
Yamanoue Y, Setiamarga D, Matsuura K (2010) Pelvic fins in teleosts: structure, function and evolution. J Fish Biol 77(6):1173–1208. https://doi.org/10.1111/j.1095-8649.2010.02674.x
Ziuganov VV, Zotin AA (1995) Pelvic girdle polymorphism and reproductive barriers in the ninespine stickleback Pungitius pungitius (L.) from northwest Russia. Behaviour 132(13/14):1095–1105. https://doi.org/10.1163/156853995X00478
Acknowledgements
The authors would like to express their sincere thanks to Frederic D. B. Schedel for collecting Kneria stappersii in the Democratic Republic of the Congo as well as Ulrich K. Schliewen and Dirk Neumann providing us with specimen of Parakneria sp. ‘mukuleshi’. Further thanks go to Ella Meißner for helping with the aquarium maintenance.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Ann-Katrin Koch and Timo Moritz equally composed the study, performed dissections, documented the results by photography and drawings, and wrote the manuscript. Ann-Katrin Koch also prepared cleared and stained specimens.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koch, AK., Moritz, T. The pelvic girdle in extant gonorynchiformes (Teleostei: Otomorpha). Zoomorphology 143, 141–150 (2024). https://doi.org/10.1007/s00435-023-00628-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00628-1