Abstract
While eye drops are the most common ocular dosage form, eye drops for treating diseases of the posterior segment (retina, choroid, optic nerve) have yet to be developed. In glaucoma, eye drops are used extensively for delivering intraocular pressure (IOP)-lowering medications to the anterior segment. However, degeneration of retinal ganglion cells (RGCs) in the retina may progress despite significant IOP lowering, suggesting that a complementary neuroprotective therapy would improve glaucoma management. Here, we describe a hypotonic, thermosensitive gel-forming eye drop for effective delivery of sunitinib, a protein kinase inhibitor with activity against the neuroprotective targets dual leucine zipper kinase (DLK) and leucine zipper kinase (LZK), to enhance survival of RGCs after optic nerve injury. Further, binding of sunitinib to melanin in the pigmented cells in the choroid and retinal pigment epithelium (RPE) led to prolonged intraocular residence time, including therapeutically relevant concentrations in the non-pigmented retinal tissue where the RGCs reside. The combination of enhanced intraocular absorption provided by the gel-forming eye drop vehicle and the intrinsic melanin binding properties of sunitinib led to significant protection of RGCs with only once weekly eye drop dosing. For a chronic disease such as glaucoma, an effective once weekly eye drop for neuroprotection could result in greater patient adherence, and thus, greater disease management and improved patient quality of life.
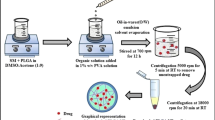
Graphical abstract





Similar content being viewed by others
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Atey TM, et al. The impact of adherence and instillation proficiency of topical glaucoma medications on intraocular pressure. J Ophthalmol. 2017;2017:1683430.
Rodrigues GA, et al. Topical drug delivery to the posterior segment of the eye: addressing the challenge of preclinical to clinical translation. Pharm Res. 2018;35(12):245.
Kim YC, et al. Gelling hypotonic polymer solution for extended topical drug delivery to the eye. Nat Biomed Eng. 2020;4(11):1053–62.
Roskoski R Jr. Sunitinib: a VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun. 2007;356(2):323–8.
Welsbie DS, et al. Enhanced functional genomic screening identifies novel mediators of dual leucine zipper kinase-dependent injury signaling in neurons. Neuron, 2017;94(6): p. 1142–1154 e6.
Welsbie DS, et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A. 2013;110(10):4045–50.
Watkins TA, et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci U S A. 2013;110(10):4039–44.
Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12(4):387–9.
Durairaj C, Chastain JE, Kompella UB. Intraocular distribution of melanin in human, monkey, rabbit, minipig and dog eyes. Exp Eye Res. 2012;98:23–7.
Rimpela AK, et al. Implications of melanin binding in ocular drug delivery. Adv Drug Deliv Rev. 2018;126:23–43.
Chen KG, et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci U S A. 2006;103(26):9903–7.
Salazar M, Patil PN. An explanation for the long duration of mydriatic effect of atropine in eye. Invest Ophthalmol. 1976;15(8):671–3.
Rimpela AK, et al. Melanin targeting for intracellular drug delivery: quantification of bound and free drug in retinal pigment epithelial cells. J Control Release. 2018;283:261–8.
Laster M, Norris KC. Lesson learned in mortality and kidney transplant outcomes among pediatric dialysis patients. J Am Soc Nephrol. 2017;28(5):1334–6.
Wolkow N, et al. Iron upregulates melanogenesis in cultured retinal pigment epithelial cells. Exp Eye Res. 2014;128:92–101.
Cheruvu NP, Amrite AC, Kompella UB. Effect of eye pigmentation on transscleral drug delivery. Invest Ophthalmol Vis Sci. 2008;49(1):333–41.
Speed B, et al. Pharmacokinetics, distribution, and metabolism of [14C]sunitinib in rats, monkeys, and humans. Drug Metab Dispos. 2012;40(3):539–55.
Rimpela AK, et al. Drug distribution to retinal pigment epithelium: studies on melanin binding, cellular kinetics, and single photon emission computed tomography/computed tomography imaging. Mol Pharm. 2016;13(9):2977–86.
Du W, et al. The effect of ocular pigmentation on transscleral delivery of triamcinolone acetonide. J Ocul Pharmacol Ther. 2013;29(7):633–8.
Ono C, Tanaka M. Binding characteristics of fluoroquinolones to synthetic levodopa melanin. J Pharm Pharmacol. 2003;55(8):1127–33.
Koeberle MJ, et al. Development of a liquid chromatography-mass spectrometric method for measuring the binding of memantine to different melanins. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787(2):313–22.
Ahmado A, et al. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Invest Ophthalmol Vis Sci. 2011;52(10):7148–59.
Hackett SF, et al. Sustained delivery of acriflavine from the suprachoroidal space provides long term suppression of choroidal neovascularization. Biomaterials. 2020;243:119935.
Goodman VL, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13(5):1367–73.
Liu Y, et al. Differential gamma-synuclein expression in acute and chronic retinal ganglion cell death in the retina and optic nerve. Mol Neurobiol. 2020;57(2):698–709.
Jiang SM, et al. Beta-III-tubulin: a reliable marker for retinal ganglion cell labeling in experimental models of glaucoma. Int J Ophthalmol. 2015;8(4):643–52.
Quigley HA. Use of animal models and techniques in glaucoma research: introduction. Methods Mol Biol. 2018;1695:1–10.
Leblanc B, et al. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul Toxicol Pharmacol. 1998;28(2):124–32.
Singh R, et al. Clinical evaluation of pazopanib eye drops in healthy subjects and in subjects with neovascular age-related macular degeneration. Retina. 2014;34(9):1787–95.
Nomoto H, et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50(10):4807–13.
Iwase T, et al. Topical pazopanib blocks VEGF-induced vascular leakage and neovascularization in the mouse retina but is ineffective in the rabbit. Invest Ophthalmol Vis Sci. 2013;54(1):503–11.
Tse AP, et al. Glaucoma treatment adherence at a United Kingdom general practice. Eye (Lond). 2016;30(8):1118–22.
Chawla A, McGalliard JN, Batterbury M. Use of eyedrops in glaucoma: how can we help to reduce non-compliance? Acta Ophthalmol Scand. 2007;85(4):464.
Aref AA. Sustained drug delivery for glaucoma: current data and future trends. Curr Opin Ophthalmol. 2017;28(2):169–74.
Black AC, et al. Latanoprost in pediatric glaucoma–pediatric exposure over a decade. J AAPOS. 2009;13(6):558–62.
Fogagnolo P, Rossetti L. Medical treatment of glaucoma: present and future. Expert Opin Investig Drugs. 2011;20(7):947–59.
Sarna T. Properties and function of the ocular melanin–a photobiophysical view. J Photochem Photobiol B. 1992;12(3):215–58.
Hu DN, Simon JD, Sarna T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem Photobiol. 2008;84(3):639–44.
Rozanowska M, et al. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic Biol Med. 1999;26(5–6):518–25.
Wakamatsu K, et al. Characterization of melanin in human iridal and choroidal melanocytes from eyes with various colored irides. Pigment Cell Melanoma Res. 2008;21(1):97–105.
Jakubiak P, et al. Establishment of an in vitro-in vivo correlation for melanin binding and the extension of the ocular half-life of small-molecule drugs. Mol Pharm. 2019;16(12):4890–901.
Pitkanen L, et al. Binding of betaxolol, metoprolol and oligonucleotides to synthetic and bovine ocular melanin, and prediction of drug binding to melanin in human choroid-retinal pigment epithelium. Pharm Res. 2007;24(11):2063–70.
Steiner K, Buhring KU, Merck E. The melanin binding of bisoprolol and its toxicological relevance. Lens Eye Toxic Res. 1990;7(3–4):319–33.
Salazar M, Shimada K, Patil PN. Iris pigmentation and atropine mydriasis. J Pharmacol Exp Ther. 1976;197(1):79–88.
Acheampong AA, Shackleton M, Tang-Liu DD. Comparative ocular pharmacokinetics of brimonidine after a single dose application to the eyes of albino and pigmented rabbits. Drug Metab Dispos. 1995;23(7):708–12.
Burke JA, et al. The role of ocular pigmentation in the intraocular pressure response to brimonidine in rabbits. Invest Ophthalmol Vis Sci. 2011;52(14):2466–2466.
Araie M, et al. Beta-adrenergic blockers: ocular penetration and binding to the uveal pigment. Jpn J Ophthalmol. 1982;26(3):248–63.
Nagata A, et al. Binding of antiglaucomatous drugs to synthetic melanin and their hypotensive effects on pigmented and nonpigmented rabbit eyes. Jpn J Ophthalmol. 1993;37(1):32–8.
Tsujinaka H, et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat Commun. 2020;11(1):694.
Coleman CI, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18(7):527–39.
Saini SD, et al. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15(6):e22-33.
Caldeira D, Vaz-Carneiro A, Costa J. The impact of dosing frequency on medication adherence in chronic cardiovascular disease: systematic review and meta-analysis. Rev Port Cardiol. 2014;33(7–8):431–7.
Laliberte F, et al. Impact of daily dosing frequency on adherence to chronic medications among nonvalvular atrial fibrillation patients. Adv Ther. 2012;29(8):675–90.
Cramer JA, et al. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21(9):1453–60.
Iglay K, et al. Systematic literature review and meta-analysis of medication adherence with once-weekly versus once-daily therapy. Clin Ther, 2015. 37(8): p. 1813–21 e1.
Cramer JA, et al. The effect of dosing frequency on compliance and persistence with bisphosphonate therapy in postmenopausal women: a comparison of studies in the United States, the United Kingdom, and France. Clin Ther. 2006;28(10):1686–94.
Qiao Q, et al. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201–5.
Ishii H, et al. Randomized multicenter evaluation of quality of life and treatment satisfaction in type 2 diabetes patients receiving once-weekly trelagliptin versus a daily dipeptidyl peptidase-4 inhibitor. Diabetes Ther. 2019;10(4):1369–80.
Ito H, et al. Changes in medication adherence and unused drugs after switching from daily dipeptidyl peptidase-4 inhibitors to once-weekly trelagliptin in patients with type 2 diabetes. Diabetes Res Clin Pract. 2019;153:41–8.
Waisbourd M, et al. The Wills Eye Glaucoma app: interest of patients and their caregivers in a smartphone-based and tablet-based glaucoma application. J Glaucoma. 2016;25(9):e787–91.
ADAGIO Study provides evidence that GB-102 can achieve sustained-drug delivery in the treatment of wet age-related macular degeneration (AMD). [cited 2020 Dec. 26th]; Available from: https://www.graybug.vision/graybug-vision-presents-top-line-results-of-phase-1-2a-adagio-study-at-hawaiian-eye-retina-2019/.
A depot formulation of sunitinib malate (GB-102) compared to aflibercept in subjects with wet AMD (ALTISSIMO) (ClinicalTrials.gov Identifier: NCT03953079). [cited 2020 Dec. 26th].
Acknowledgements
The authors thank the veterinary and husbandry staff for their assistance and Fareeha Zulfiqar for administrative support. The authors thank the veterinary and husbandry staff for their assistance and Fareeha Zulfiqar for administrative support.
Funding
This work was supported by the National Institutes of Health (R01EY031041, R01EY026578 and P30EY001765), the Robert H. Smith Family Foundation, a Sybil B. Harrington Special Scholar Award and a departmental grant from Research to Prevent Blindness, the KKESH—WEI Collaborative Research Fund, a Hartwell Foundation Postdoctoral Fellowship, and the Guerrieri Family Research Fund. Drug measurements were conducted by the Analytical Pharmacology Core (APC) of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. The work conducted by the APC was supported by NIH grants P30CA006973 and S10OD020091, as well as grant number UL1TR003098 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Author information
Authors and Affiliations
Contributions
Conceptualization: L.M.E, J.H., D.J.Z., Y.C.K. Data collection and analysis: H.T.H., M.D.S., C.A.B., H.H., N.M.A., A.H., K.T.L., R.T.C., H.K., M.B.A., U.R., P.K., C.E., I.P. Writing and editing: L.M.E., Y.C.K., H.T.H., N.M.A., I.P., D.J.Z. All the authors read and approved of the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All institutional and national guidelines for the care and use of laboratory animals were followed.
Consent for publication
All authors give consent for publication.
Competing interests
Y.C.K., L.M.E., H.T.H., and J.H. are inventors on patents/patent applications related to this technology. J.H. and L.M.E. are co-founders of a start-up company planning to develop the eye drop technology for clinical use. DJZ and CAB are inventors on patents related to sunitinib and neuroprotection. Some of these patents have been licensed to Graybug Vision and to Oriole Therapeutics. J.H. is a co-founder and equity owner of Graybug Vision, and both J.H. and Johns Hopkins own company stock. DJZ is a co-founder and equity owner in Oriole Therapeutics. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. All other authors declare that they have no competing interests.
Disclaimer
The contents is solely the responsibility of the authors and does not necessarily represent the official view of the NCATS or NIH.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, Y.C., Hsueh, H.T., Shin, M.D. et al. A hypotonic gel-forming eye drop provides enhanced intraocular delivery of a kinase inhibitor with melanin-binding properties for sustained protection of retinal ganglion cells. Drug Deliv. and Transl. Res. 12, 826–837 (2022). https://doi.org/10.1007/s13346-021-00987-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00987-6