Abstract
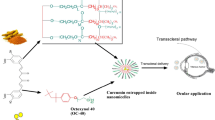
The objective of this study was to develop a clear, aqueous rapamycin-loaded mixed nanomicellar formulations (MNFs) for the back-of-the-eye delivery. MNF of rapamycin (0.2%) was prepared with vitamin E tocopherol polyethylene glycol succinate (TPGS) (Vit E TPGS) and octoxynol-40 (Oc-40) as polymeric matrix. MNF was characterized by various parameters such as size, charge, shape, and viscosity. Proton nuclear magnetic resonance (1H NMR) was used to identify unentrapped rapamycin in MNF. Cytotoxicity was evaluated in human retinal pigment epithelial (D407) and rabbit primary corneal epithelial cells (rPCECs). In vivo posterior ocular rapamycin distribution studies were conducted in male New Zealand white rabbits. The optimized MNF has excellent rapamycin entrapment and loading efficiency. The average size of MNF was 10.98 ± 0.089 and 10.84 ± 0.11 nm for blank and rapamycin-loaded MNF, respectively. TEM analysis revealed that nanomicelles are spherical in shape. Absence of free rapamycin in the MNF was confirmed by 1H NMR studies. Neither placebo nor rapamycin-loaded MNF produced cytotoxicity on D407 and rPCECs indicating formulations are tolerable. In vivo studies demonstrated a very high rapamycin concentration in retina-choroid (362.35 ± 56.17 ng/g tissue). No drug was identified in the vitreous humor indicating the sequestration of rapamycin in lipoidal retinal tissues. In summary, a clear, aqueous MNF comprising of Vit E TPGS and Oc-40 loaded with rapamycin was successfully developed. Back-of-the-eye tissue distribution studies demonstrated a very high rapamycin levels in retina-choroid (place of drug action) with a negligible drug partitioning into vitreous humor.









Similar content being viewed by others
References
Nguyen QD, Ibrahim MA, Watters A, Bittencourt M, Yohannan J, Sepah YJ, et al. Ocular tolerability and efficacy of intravitreal and subconjunctival injections of sirolimus in patients with non-infectious uveitis: primary 6-month results of the SAVE Study. J ophthalmic Inflamm Infect. 2013;3(1):32. PubMed PMID: 23514595Pubmed Central PMCID: 3610181.
Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008;42(1–3):41–50. PubMed PMID: 18629448 Pubmed Central PMCID: 2756228.
Gupta R, Murray PI. Chronic non-infectious uveitis in the elderly: epidemiology, pathophysiology and management. Drugs Aging. 2006;23(7):535–58. PubMed PMID: 16930083.
Pato E, Munoz-Fernandez S, Francisco F, Abad MA, Maese J, Ortiz A, et al. Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis. Semin Arthritis Rheum. 2011;40(4):314–23. PubMed PMID: 20656330.
Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159–62. PubMed PMID: 15317708 Pubmed Central PMCID: 1772296.
Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J ocul pharmaco ther J Assoc Ocul Pharmacol Ther. 2013;29(2):106–23. PubMed PMID: 23215539 Pubmed Central PMCID: 3601677.
Bian ZM, Elner SG, Elner VM. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res. 2007;84(5):812–22. PubMed PMID: 17331500 Pubmed Central PMCID: 2094015.
Khurana RN, Do DV, Nguyen QD. Anti-VEGF therapeutic approaches for diabetic macular edema. Int Ophthalmol Clin. 2009;49(2):109–19. PubMed PMID: 19349791.
Kabeel MM, El-Batarny AM, Tameesh MK, Abou El Enein MA. Combined intravitreal bevacizumab and photodynamic therapy with vertiporfin for management of choroidal neovascularization secondary to age-related macular degeneration. Clin Ophthalmol. 2008;2(1):159–66. PubMed PMID: 19668400Pubmed Central PMCID: 2698683.
Blumenkranz MS, Woodburn KW, Qing F, Verdooner S, Kessel D, Miller R. Lutetium texaphyrin (Lu-Tex): a potential new agent for ocular fundus angiography and photodynamic therapy. Am J Ophthalmol. 2000;129(3):353–62. PubMed PMID: 10704552.
Wu L, Murphy RP. Photodynamic therapy: a new approach to the treatment of choroidal neovascularization secondary to age-related macular degeneration. Curr Opin Ophthalmol. 1999;10(3):217–20. PubMed PMID: 10537782.
Stahl A, Paschek L, Martin G, Gross NJ, Feltgen N, Hansen LL, et al. Rapamycin reduces VEGF expression in retinal pigment epithelium (RPE) and inhibits RPE-induced sprouting angiogenesis in vitro. FEBS Lett. 2008;582(20):3097–102. PubMed PMID: 18703055.
Salzmann J, Lightman S. The potential of newer immunomodulating drugs in the treatment of uveitis: a review BioDrugs clinical immunotherapeutics. Biopharmaceuticals gene ther. 2000;13(6):397–408. PubMed PMID: 18034546.
Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31(5):335–40. PubMed PMID: 9721431.
Terada N, Lucas JJ, Szepesi A, Franklin RA, Domenico J, Gelfand EW. Rapamycin blocks cell cycle progression of activated T cells prior to events characteristic of the middle to late G1 phase of the cycle. J Cell Physiol. 1993;154(1):7–15. PubMed PMID: 8419408.
Wong GK, Griffith S, Kojima I, Demain AL. Antifungal activities of rapamycin and its derivatives, prolylrapamycin, 32-desmethylrapamycin, and 32-desmethoxyrapamycin. J antibiotics. 1998;51(5):487–91. PubMed PMID: 9666177.
Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–35. PubMed PMID: 11821896.
Dejneka NS, Kuroki AM, Fosnot J, Tang W, Tolentino MJ, Bennett J. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol Vis. 2004;10:964–72. PubMed PMID: 15623986.
Rouf MA, Vural I, Renoir JM, Hincal AA. Development and characterization of liposomal formulations for rapamycin delivery and investigation of their antiproliferative effect on MCF7 cells. J liposome res. 2009;19(4):322–31. PubMed PMID: 19863167.
Buech G, Bertelmann E, Pleyer U, Siebenbrodt I, Borchert HH. Formulation of sirolimus eye drops and corneal permeation studies. J ocul pharmaco ther J Assoc Ocul Pharmacol Ther. 2007;23(3):292–303. PubMed PMID: 17593014.
Trepanier DJ, Gallant H, Legatt DF, Yatscoff RW. Rapamycin: distribution, pharmacokinetics and therapeutic range investigations: an update. Clin Biochem. 1998;31(5):345–51. PubMed PMID: 9721433.
Yatscoff RW, Wang P, Chan K, Hicks D, Zimmerman J. Rapamycin: distribution, pharmacokinetics, and therapeutic range investigations. Ther Drug Monit. 1995;17(6):666–71. PubMed PMID: 8588238.
Zhaoliang Zhang LX, Hao Chen, Xingyi Li. Rapamycin-loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) nanoparticles: preparation, characterization and potential application in corneal transplantation. Journal of Pharmacy and Pharmacology. In press.
Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J controlled release j Controlled Release Soc. 2006;110(2):370–7. PubMed PMID: 16298448.
Mu L, Feng SS. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol). J controlled release J Controlled Release Soc. 2002;80(1–3):129–44. PubMed PMID: 11943393.
Eva-Maria C, Christiane B, Wempe MF, John H, Lisa N, Edgar KJ, et al. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J Control Release. 2006;111(1–2):35–40.
Collnot EM, Baldes C, Wempe MF, Kappl R, Huttermann J, Hyatt JA, et al. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm. 2007;4(3):465–74. PubMed PMID: 17367162.
Khandavilli S, Panchagnula R. Nanoemulsions as versatile formulations for paclitaxel delivery: peroral and dermal delivery studies in rats. J investigative dermatol. 2007;127(1):154–62. PubMed PMID: 16858422.
Cholkar K, Hariharan S, Gunda S, Mitra AK. Optimization of dexamethasone mixed nanomicellar formulation. AAPS PharmSciTech. 2014 Jul 1. PubMed PMID: 24980081
Vadlapudi AD, Cholkar K, Vadlapatla RK, Mitra AK. Aqueous nanomicellar formulation for topical delivery of biotinylated lipid prodrug of acyclovir: formulation development and ocular biocompatibility. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013 Nov 5. PubMed PMID: 24192229
Hariharan S, Minocha M, Mishra GP, Pal D, Krishna R, Mitra AK. Interaction of ocular hypotensive agents (PGF2 alpha analogs-bimatoprost, latanoprost, and travoprost) with MDR efflux pumps on the rabbit cornea. J ocul pharmaco ther J Assoc Ocul Pharmacol Ther. 2009;25(6):487–98. PubMed PMID: 20028257Pubmed Central PMCID: 3096535.
Dong XY, Feng XD, Sun Y. His-tagged protein purification by metal-chelate affinity extraction with nickel-chelate reverse micelles. Biotechnol Prog. 2010;26(4):1088–94. PubMed PMID: 20730766.
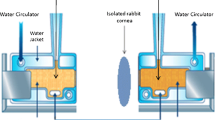
Earla R, Cholkar K, Gunda S, Earla RL, Mitra AK. Bioanalytical method validation of rapamycin in ocular matrix by QTRAP LC-MS/MS: application to rabbit anterior tissue distribution by topical administration of rapamycin nanomicellar formulation. J Chromatogr B Anal Technol Biomed Life Sci. 2012;908:76–86. PubMed PMID: 23122404Pubmed Central PMCID: 3597233.
accessed on December, 21 of 2013. Available from: http://www.accessdata.fda.gov/scripts/cder/iig/getiigWEB.cfm.
Zhu H, Chauhan A. Effect of viscosity on tear drainage and ocular residence time. Optometry and vision science American Academy of Optometry. 2008;85(8):715–25. PubMed PMID: 18677227.
Benjamin WJ, Hill RM. Tonicity of human tear fluid sampled from the cul-de-sac. Br J Ophthalmol. 1989;73(8):624–7. PubMed PMID: 2765441Pubmed Central PMCID: 1041831.
Abelson MB, Udell IJ, Weston JH. Normal human tear pH by direct measurement. Arch Ophthalmol. 1981;99(2):301. PubMed PMID: 7469869.
Chopra P, Hao J, Li SK. Iontophoretic transport of charged macromolecules across human sclera. Int J Pharm. 2010;388(1–2):107–13. PubMed PMID: 20045044Pubmed Central PMCID: 2838613.
Wei Z, Hao J, Yuan S, Li Y, Juan W, Sha X, et al. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376(1–2):176–85. PubMed PMID: 19409463.
Lee SC, Huh KM, Lee J, Cho YW, Galinsky RE, Park K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: in vitro and in vivo characterization. Biomacromolecules. 2007;8(1):202–8. PubMed PMID: 17206808Pubmed Central PMCID: 2532792.
Renliang Xu MAW, Hallett FR, Gerard R, Croucher MD. Light-scattering study of the association behavior of styrene-ethylene oxide block copolymers in aqueous solution. Macromolecules. 1991;24(1):87–93.
Kristiina Järvinena TJ. Arto urtti. Ocular absorption following topical delivery Adv Drug Deliv Rev. 1995;16(1):3–19.
Hughes PM, Oleinik O, Joan-En Chang L, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57(14):2010–32.
Aydemir O, Celebi S, Yilmaz T, Yekeler H, Kukner AS. Protective effects of vitamin E forms (alpha-tocopherol, gamma-tocopherol and d-alpha-tocopherol polyethylene glycol 1000 succinate) on retinal edema during ischemia-reperfusion injury in the guinea pig retina. Int Ophthalmol. 2004;25(5–6):283–9. PubMed PMID: 16532291.
Dugel PU. Sirolimus in treatment of retinal diseases. Retina today. 2009 (October):38–41.
Anglicheau D, Pallet N, Rabant M, Marquet P, Cassinat B, Meria P, et al. Role of P-glycoprotein in cyclosporine cytotoxicity in the cyclosporine-sirolimus interaction. Kidney Int. 2006;70(6):1019–25. PubMed PMID: 16837925.
accessed on 31st December 2013. Available from: http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm.
Collnot EM, Baldes C, Schaefer UF, Edgar KJ, Wempe MF, Lehr CM. Vitamin E TPGS P-glycoprotein inhibition mechanism: influence on conformational flexibility, intracellular ATP levels, and role of time and site of access. Mol Pharm. 2010;7(3):642–51. PubMed PMID: 20205474.
Acknowledgments
This study was supported by NIH grants R01EY09171-16 and R01EY010659-14. We would like to thank Dr. Vladimir Dusevich, UMKC School of Dentistry, for helping with the operation of transmission electron microscopy. The authors also acknowledge LUX Biosciences, NJ, USA, for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cholkar, K., Gunda, S., Earla, R. et al. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS PharmSciTech 16, 610–622 (2015). https://doi.org/10.1208/s12249-014-0244-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0244-2