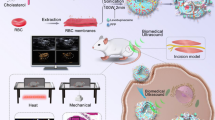
Abstract
The effect of local anesthetics, particularly those which are hydrophilic, such as tetrodotoxin, is impeded by tissue barriers that restrict access to individual nerve cells. Methods of enhancing penetration of tetrodotoxin into nerve include co-administration with chemical permeation enhancers, nanoencapsulation, and insonation with very low acoustic intensity ultrasound and microbubbles. In this study, we examined the effect of acoustic intensity on nerve block by tetrodotoxin and compared it to the effect on nerve block by bupivacaine, a more hydrophobic local anesthetic. Anesthetics were applied in peripheral nerve blockade in adult Sprague-Dawley rats. Insonation with 1-MHz ultrasound at acoustic intensity greater than 0.5 W/cm2 improved nerve block effectiveness, increased nerve block reliability, and prolonged both sensory and motor nerve blockade mediated by the hydrophilic ultra-potent local anesthetic, tetrodotoxin. These effects were not enhanced by microbubbles. There was minimal or no tissue injury from ultrasound treatment. Insonation did not enhance nerve block from bupivacaine. Using an in vivo model system of local anesthetic delivery, we studied the effect of acoustic intensity on insonation-mediated drug delivery of local anesthetics to the peripheral nerve. We found that insonation alone (at intensities greater than 0.5 W/cm2) enhanced nerve blockade mediated by the hydrophilic ultra-potent local anesthetic, tetrodotoxin.

Graphical abstract





Similar content being viewed by others
Abbreviations
- TTX:
-
Tetrodotoxin
- S1SCB:
-
Site-one sodium channel blocker
- PBS:
-
Phosphate-buffered saline
- CPE:
-
Chemical permeation enhancer
References
Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. J Clin Anesth. 2016;35:524–9.
Piña-Oviedo S, Ortiz-Hidalgo C. The normal and neoplastic perineurium: a review. Adv Anat Pathol. 2008;15:147–64.
Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89:119–31.
Kohane DS, Lu NT, Gökgöl-Kline AC, Shubina M, Kuang Y, Hall S, et al. The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo. Reg Anesth Pain Med. 2000;25:52–9.
Hille B. The receptor for tetrodotoxin and saxitoxin. A structural hypothesis. Biophys J. 1975;15:615–9.
Rodriguez-Navarro AJ, Lagos N, Lagos M, Braghetto I, Csendes A, Hamilton J, et al. Neosaxitoxin as a local anesthetic: preliminary observations from a first human trial. Anesthesiology. 2007;106:339–45.
Lobo K, Donado C, Cornelissen L, Kim J, Ortiz R, Peake RWA, et al. A phase 1, dose-escalation, double-blind, block-randomized, controlled trial of safety and efficacy of neosaxitoxin alone and in combination with 0.2% bupivacaine, with and without epinephrine, for cutaneous anesthesia. Anesthesiology. 2015;123:873–85.
Simons EJ, Bellas E, Lawlor MW, Kohane DS. Effect of chemical permeation enhancers on nerve blockade. Mol Pharm. 2009;6:265–73.
Santamaria CM, Zhan C, McAlvin JB, Zurakowski D, Kohane DS. Tetrodotoxin, epinephrine, and chemical permeation enhancer combinations in peripheral nerve blockade. Anesth. Analg. Anesthesia and analgesia; 2017;1.
Liu Q, Santamaria CM, Wei T, Zhao C, Ji T, Yang T, et al. Hollow silica nanoparticles penetrate the peripheral nerve and enhance the nerve blockade from tetrodotoxin. Nano Lett. 2017;18:32–7.
Oberli MA, Schoellhammer CM, Langer R, Blankschtein D. Ultrasound-enhanced transdermal delivery: recent advances and future challenges. Ther Deliv. 6 ed. Future Science Ltd London, UK; 2014;5:843–57.
Yang R, Wei T, Goldberg H, Wang W, Cullion K, Kohane DS. Getting drugs across biological barriers. Adv Mater. 2017;29:1606596.
Ahmadi F, McLoughlin IV, Chauhan S, ter-Haar G. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog Biophys Mol Biol 2012;108:119–138.
Cullion K, Santamaria CM, Zhan C, Zurakowski D, Sun T, Pemberton GL, et al. High-frequency, low-intensity ultrasound and microbubbles enhance nerve blockade. J Control Release. 2018;276:150–6.
Tiwari SB, Pai RM, Udupa N. Influence of ultrasound on the percutaneous absorption of ketorolac tromethamine in vitro across rat skin. Drug Deliv. 2004;11:47–51.
Qin S, Caskey CF, Ferrara KW. Ultrasound contrast microbubbles in imaging and therapy: physical principles and engineering. Phys Med Biol IOP Publishing; 2009;54:R27–57.
Shin J, Kong C, Cho JS, Lee J, Koh CS, Yoon M-S, et al. Focused ultrasound-mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters. Neurosurg Focus American Association of Neurological Surgeons; 2018;44:E15.
Kohane DS, Sankar WN, Shubina M, Hu D, Rifai N, Berde CB. Sciatic nerve blockade in infant, adolescent, and adult rats: a comparison of ropivacaine with bupivacaine. Anesthesiology. 1998;89:1199–208 discussion10A.
Azagury A, Amar-Lewis E, Yudilevitch Y, Isaacson C, Laster B, Kost J. Ultrasound effect on cancerous versus non-cancerous cells. Ultrasound Med Biol. 2016;42:1560–7.
Guan L, Xu G. Damage effect of high-intensity focused ultrasound on breast cancer tissues and their vascularities. World J Surg Oncol. 2016;14:153.
Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82:1013–25.
Rwei AY, Paris JL, Wang B, Wang W, Axon CD, Vallet-Regí M, et al. Ultrasound-triggered local anaesthesia. Nat Biomed Eng Nature Publishing Group; 2017;1:644–53.
Padera R, Bellas E, Tse JY, Hao D, Kohane DS. Local myotoxicity from sustained release of bupivacaine from microparticles. Anesthesiology The American Society of Anesthesiologists; 2008;108:921–8.
Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery--a general review. Expert Opin Drug Deliv Taylor & Francis; 2004;1:37–56.
Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–8.
Yang R, Saarinen R, Okonkwo OS, Hao Y, Mehta M, Kohane DS. Transtympanic delivery of local anesthetics for pain in acute otitis media. Mol Pharm. 2019;16:1555–62.
Tzu-Yin W, Wilson KE, Machtaler S, Willmann JK. Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical implications. Curr Pharm Biotechnol. 2013;14:743–52.
Suen W-LL, Wong HS, Yu Y, Lau LCM, Lo AC-Y, Chau Y. Ultrasound-mediated transscleral delivery of macromolecules to the posterior segment of rabbit eye in vivo. Invest Ophthalmol Vis Sci The Association for Research in Vision and Ophthalmology; 2013;54:4358–65.
Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. Bush AI, editor. PLoS ONE. 2008;3:e2175.
Ting C-Y, Fan C-H, Liu H-L, Huang C-Y, Hsieh H-Y, Yen T-C, et al. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33:704–12.
Mitragotri S, Edwards DA, Blankschtein D, Langer R. A mechanistic study of ultrasonically-enhanced transdermal drug delivery. J Pharm Sci. 1995;84:697–706.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8.
Newman CMH, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14:465–75.
Panje CM, Wang DS, Willmann JK. Ultrasound and microbubble-mediated gene delivery in cancer: progress and perspectives. Investig Radiol. 2013;48:755–69.
Yoon CS, Park JH. Ultrasound-mediated gene delivery. Expert Opin Drug Deliv. Taylor & Francis; 2010;7:321–30.
Thomas RG, Jonnalagadda US, Kwan JJ. Biomedical applications for gas-stabilizing solid cavitation agents. Langmuir American Chemical Society; 2019;35:10106–15.
Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38:1716–25.
Fujii H, Matkar P, Liao C, Rudenko D, Lee PJ, Kuliszewski MA, et al. Optimization of ultrasound-mediated anti-angiogenic cancer gene therapy. Mol Ther Nucleic Acids. 2013;2:e94.
Chen S, Ding J-H, Bekeredjian R, Yang B-Z, Shohet RV, Johnston SA, et al. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. PNAS. 2006;103:8469–74.
US EPA; Jan. 2019, Estimation Program Interface (EPI) Suite™ for Microsoft® Windows, v4.11. United States Environmental Protection Agencym, Washington, DC, USA.
Hansch C, Leo A, Hoekman D. Exploring QSAR: hydrophobic, electronic, steric constants. Washington, DC: American Chemical Society; 1995.
Morishita K, Karasuno H, Yokoi Y, Morozumi K, Ogihara H, Ito T. Effects of therapeutic ultrasound on intramuscular blood circulation and oxygen dynamics. Journal of the Japanese Physical Therapy Association 2014;17:1–7.
Maxwell AD, Cain CA, Duryea AP, Yuan L, Gurm HS, Xu Z. Noninvasive thrombolysis using pulsed ultrasound cavitation therapy - histotripsy. Ultrasound Med Biol. 2009;35:1982–94.
Foley JL, Little JW, Vaezy S. Effects of high-intensity focused ultrasound on nerve conduction. Muscle nerve, Wiley Subscription Services, Inc, A Wiley Company; 2008;37:241–50.
Foley JL, Little JW, Starr FL, Frantz C, Vaezy S. Image-guided HIFU neurolysis of peripheral nerves to treat spasticity and pain. Ultrasound Med Biol. 2004;30:1199–207.
Foley JL, Little JW, Vaezy S. Image-guided high-intensity focused ultrasound for conduction block of peripheral nerves. Annals of biomedical engineering Kluwer Academic Publishers-Plenum Publishers; 2006;35:109–19.
Warden SJ. A new direction for ultrasound therapy in sports medicine. Sports Med Springer International Publishing; 2003;33:95–107.
Noble JG, Lee V, Griffith-Noble F. Therapeutic ultrasound: the effects upon cutaneous blood flow in humans. Ultrasound Med Biol. 2007;33:279–85.
Fabrizio PA, Schmidt JA, Clemente FR, Lankiewicz LA, Levine ZA. Acute effects of therapeutic ultrasound delivered at varying parameters on the blood flow velocity in a muscular distribution artery. J Orthop Sports Phys Ther. JOSPT, Inc. JOSPT, 1033 North Fairfax Street, Suite 304, Alexandria, VA 22134–1540; 1996;24:294–302.
Sundaram J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J. 2003;84:3087–101.
Zhou B, Leung BYK, Sun L. The effects of low-intensity ultrasound on fat reduction of rat model. Biomed Res Int Hinedawi; 2017;2017:4701481–8.
Mehier-Humbert S, Bettinger T, Yan F, Guy RH. Plasma membrane poration induced by ultrasound exposure: implication for drug delivery. J Control Release. 2005;104:213–22.
Funding
This research was funded by the National Institutes of Health R35 GM131728 (to DSK).
Author information
Authors and Affiliations
Contributions
K.C. and D.S.K. designed the research, analyzed the data, and wrote the manuscript. K.C., L.C.P., C.M.S., T.S. G.L.P, and N.M. performed the experimentation. All authors read the manuscript and contributed to its final presentation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1611 kb)
Rights and permissions
About this article
Cite this article
Cullion, K., Petishnok, L.C., Sun, T. et al. Local anesthesia enhanced with increasing high-frequency ultrasound intensity. Drug Deliv. and Transl. Res. 10, 1507–1516 (2020). https://doi.org/10.1007/s13346-020-00760-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00760-1