Abstract
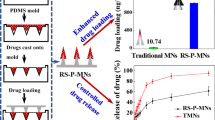
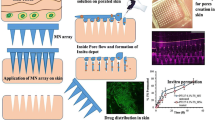
The rapidly separating microneedles (RS-PP-MNs), composed of PVA (separable arrow head) MNs and a poly(L-lactide-co-D, L-lactide) (PLA) supporting array, are used for transdermal delivery system at high humidity. The fabricated RS-PP-MNs should have sufficient mechanical strength at different humidity. In general, the water adsorption rate was increased with increasing humidity; by contrast, storage time was decreased with increasing humidity. The higher water adsorption rate indicated the lower mechanical strength, thereby lowering drug delivery efficiency. The prepared RS-PP-MNs could be successfully inserted within the skin at high humid atmosphere due to PLA supporting array. The bright field and fluorescence microscopic images suggested the probable real-time applicability of RS-PP-MNs. The in vitro and in vivo assay suggested that RS-PP-MNs potentially were able to deliver the drugs at high humidity condition. The significant improvement in the drug delivery efficiency and skin penetration ability was observed compared with the traditional MNs. In addition, the fabrication of RS-PP-MNs is facile and scalable. Therefore, the prepared RS-PP-MNs with supporting solid PLA array might be advantageous in real-time applications. This study is of great importance for the MN field as it offers more theoretical support for clinical applications.






Similar content being viewed by others
References
Shah UU, Roberts M, Gul MO, Tuleu C, Beresford MW. Needle-free and microneedle drug delivery in children: a case for disease-modifying antirheumatic drugs (DMARDs). Int J Pharm. 2011;416:1–11.
Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11–29.
Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–68.
van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161:645–55.
Cha KJ, Kim T, Park SJ, Kim DS. Simple and cost-effective fabrication of solid biodegradable polymer microneedle arrays with adjustable aspect ratio for transdermal drug delivery using acupuncture microneedles. J Micromech Microeng. 2014;24:8.
Li QY, Zhang JN, Chen BZ, Wang QL, Guo XD. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017;7:15408–15.
Chen Y, Chen BZ, Wang QL, Jin X, Guo XD. Fabrication of coated polymer microneedles for transdermal drug delivery. J Control Release. 2017;
Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–37.
Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–80.
Wang QL, Zhang XP, Chen BZ, Guo XD. Dissolvable layered microneedles with core-shell structures for transdermal drug delivery. Mater Sci Eng C. 2018;83:143–7.
Wang QL, Zhu DD, Liu XB, Chen BZ, Guo XD. Microneedles with controlled bubble sizes and drug distributions for efficient transdermal drug delivery. Sci Rep. 2016;6:38755.
Lee JW, Choi SO, Felner EI, Prausnitz MR. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small. 2011;7:531–9.
Martanto W, Moore JS, Kashlan O, Kamath R, Wang PM, O'Neal JM, et al. Microinfusion using hollow microneedles. Pharm Res. 2006;23:104–13.
Khanna P, Luongo K, Strom JA, Bhansali S. Sharpening of hollow silicon microneedles to reduce skin penetration force. J Micromech Microeng. 2010;20:8.
Vinayakumar KB, Kulkarni PG, Nayak MM, Dinesh NS, Hegde GM, Ramachandra SG, et al. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J Micromech Microeng. 2016;26:9.
Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–24.
Chu LY, Choi S-O, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci. 2010;99:4228–38.
Lee K, Lee CY, Jung H. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose. Biomaterials. 2011;32:3134–40.
Liu S, Jin M-N, Quan Y-s, Kamiyama F, Kusamori K, Katsumi H, Sakane T, Yamamoto A Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur J Pharm Biopharm 2014;86:267–276.
Zhu Z, Luo H, Lu W, Luan H, Wu Y, Luo J, et al. Rapidly dissolvable microneedle patches for transdermal delivery of exenatide. Pharm Res. 2014;31:3348–60.
Chu LY, Prausnitz MR. Separable arrowhead microneedles. J Control Release. 2011;149:242–9.
Chen MC, Huang SF, Lai KY, Ling MH. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials. 2013;34:3077–86.
Zhu DD, Chen BZ, He MC, Guo XD. Structural optimization of rapidly separating microneedles for efficient drug delivery. J Ind Eng Chem. 2017;51:178–84.
Zhu DD, Wang QL, Liu XB, Guo XD. Rapidly separating microneedles for transdermal drug delivery. Acta Biomater. 2016;41:312–9.
Wang QL, Ren JW, Chen BZ, Jin X, Zhang CY, Guo XD. Effect of humidity on mechanical properties of dissolving microneedles for transdermal drug delivery. J Ind Eng Chem. 2017
Park J-H, Yoon Y-K, Choi S-O, Prausnitz MR, Allen MG. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans Biomed Eng. 2007;54:903–13.
Verbaan FJ, Bal SM, van den Berg DJ, Dijksman JA, van Hecke M, Verpoorten H, et al. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J Control Release. 2008;128:80–8.
Funding
This work was financially supported by the National Natural Science Foundation of China (51673019, 51473017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the animal studies were conducted in accordance with the guidelines of the Ethics Committee of Beijing University of Chemical Technology (BUCT), Beijing, China
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
He, M.C., Chen, B.Z., Ashfaq, M. et al. Assessment of mechanical stability of rapidly separating microneedles for transdermal drug delivery. Drug Deliv. and Transl. Res. 8, 1034–1042 (2018). https://doi.org/10.1007/s13346-018-0547-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0547-z