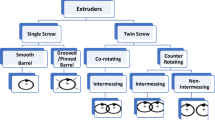
Abstract
Most of published reviews of twin-screw extrusion focused on its application for enhancing the bioavailability of amorphous solid dispersions while few of them focused on its use for manufacturing sustained-release oral dosage forms and medical implants, despite the considerable interest and success this process has garnered both in academia and in the pharmaceutical industry. Compared to conventional batch processing, twin-screw extrusion offers the advantages of continuous processing and the ability to prepare oral dosage forms and medical implants that have unique physicochemical and drug release attributes. This review provides an in-depth analysis of the formulation composition and processing conditions of twin-screw extrusion and how these factors affect the drug release properties of sustained-release dosage forms. This review also illustrates the unique advantages of this process by presenting case studies of a wide variety of commercial sustained-release products manufactured using twin-screw extrusion.











Similar content being viewed by others
References
Mohr W, Saxton R, Jepson C. Theory of mixing in the single-screw extruder. Ind Eng Chem. 1957;49(11):1857–62. https://doi.org/10.1021/ie50575a031.
El-Egakey MA, Soliva M, Speiser P. Hot extruded dosage forms. I. Technology and dissolution kinetics of polymeric matrices. Pharm Acta Helv. 1971;46(1):31–52.
Ghebre-Sellassie I, Reisch R, Parikh R, Fazwi MB, Nesbitt RU, inventors; WarnerLambert Company, assignee. Solid Pharmaceutical Dispersions Patent 6,677,362. 2004.
Breitenbach J. Melt extrusion can bring new benefits to HIV therapy. Am J Drug Deliv. 2006;4(2):61–4. https://doi.org/10.2165/00137696-200604020-00001.
Klein CE, Chiu YL, Awni W, Zhu T, Heuser RS, Doan T, et al. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. Jaids-J Acquir Immune Defic Syndr. 2007;44(4):401–10.
Singh R, Ierapetritou M, Ramachandran R. System-wide hybrid MPC-PID control of a continuous pharmaceutical tablet manufacturing process via direct compaction. Eur J Pharm Biopharm. 2013;85(3 Pt B):1164–82.
Gryczke A, Schminke S, Maniruzzaman M, Beck J, Douroumis D. Development and evaluation of orally disintegrating tablets (ODTs) containing ibuprofen granules prepared by hot melt extrusion. Colloids Surf B: Biointerfaces. 2011;86(2):275–84. https://doi.org/10.1016/j.colsurfb.2011.04.007.
Follonier N, Doelker E, Cole E. Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained-release capsules containing high loading of freely water soluble drugs. Drug Dev Ind Pharm. 1994;20(8):1323–39. https://doi.org/10.3109/03639049409038373.
Alshetaili AS, Almutairy BK, Alshahrani SM, Ashour EA, Tiwari RV, Alshehri SM, et al. Optimization of hot melt extrusion parameters for sphericity and hardness of polymeric face-cut pellets. Drug Dev Ind Pharm. 2016;42(11):1833–41. https://doi.org/10.1080/03639045.2016.1178769.
Alshetaili AS, Almutairy BK, Tiwari RV, Morott JT, Alshehri SM, Feng X, et al. Preparation and evaluation of hot-melt extruded patient-centric ketoprofen mini-tablets. Curr Drug Deliv. 2016;13(5):730–41. https://doi.org/10.2174/1567201812666151012113806.
De Brabander C, Vervaet C, Fiermans L, Remon JP. Matrix mini-tablets based on starch/microcrystalline wax mixtures. Int J Pharm. 2000;199(2):195–203. https://doi.org/10.1016/S0378-5173(00)00383-5.
Zhang F, McGinity JW. Properties of sustained-release tablets prepared by hot-melt extrusion. Pharm Dev Technol. 1999;4(2):241–50. https://doi.org/10.1081/PDT-100101358.
Pimparade MB, Vo A, Maurya AS, Bae J, Morott JT, Feng X, et al. Development and evaluation of an oral fast disintegrating anti-allergic film using hot-melt extrusion technology. Eur J Pharm Biopharm. 2017;119:81–90. https://doi.org/10.1016/j.ejpb.2017.06.004.
Ghalanbor Z, Korber M, Bodmeier R. Protein release from poly(lactide-co-glycolide) implants prepared by hot-melt extrusion: thioester formation as a reason for incomplete release. Int J Pharm. 2012;438(1–2):302–6. https://doi.org/10.1016/j.ijpharm.2012.09.015.
Meuser F, Gimmler N, Van Lengerich B. A systems analytical approach to extrusion. In: Ho CTH, Karwe MV, Kokini JL, editors. Food extrusion science and technology. New York: Marcel Dekker; 1992. p. 619–30.
Sun CC. Decoding powder tabletability: roles of particle adhesion and plasticity. J Adhes Sci Technol. 2011;25(4–5):483–99. https://doi.org/10.1163/016942410X525678.
Almeida A, Possemiers S, Boone MN, De Beer T, Quinten T, Van Hoorebeke L, et al. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur J Pharm Biopharm. 2011;77(2):297–305. https://doi.org/10.1016/j.ejpb.2010.12.004.
Claeys B, Vervaeck A, Hillewaere XKD, Possemiers S, Hansen L, De Beer T, et al. Thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding. Eur J Pharm Biopharm. 2015;90:44–52. https://doi.org/10.1016/j.ejpb.2014.11.003.
Aoki H, Iwao Y, Uchimoto T, Noguchi S, Kajihara R, Takahashi K, et al. Fine granules showing sustained drug release prepared by high-shear melt granulation using triglycerin full behenate and milled microcrystalline cellulose. Int J Pharm. 2015;478(2):530–9. https://doi.org/10.1016/j.ijpharm.2014.11.058.
Aoki H, Iwao Y, Mizoguchi M, Noguchi S, Itai S. Clarithromycin highly-loaded gastro-floating fine granules prepared by high-shear melt granulation can enhance the efficacy of helicobacter pylori eradication. Eur J Pharm Biopharm. 2015;92:22–7. https://doi.org/10.1016/j.ejpb.2015.02.012.
Carley JF. Fundamentals of melt rheology, heat generation, and heat transfer as applied to polymer processing. Polym Eng Sci. 1966;6(2):158–68. https://doi.org/10.1002/pen.760060213.
Schaefer T, Holm P, Kristensen H. Melt granulation in a laboratory scale high shear mixer. Drug Dev Ind Pharm. 1990;16(8):1249–77. https://doi.org/10.3109/03639049009115960.
Passerini N, Calogerà G, Albertini B, Rodriguez L. Melt granulation of pharmaceutical powders: a comparison of high-shear mixer and fluidised bed processes. Int J Pharm. 2010;391(1):177–86. https://doi.org/10.1016/j.ijpharm.2010.03.013.
Liu JP, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur J Pharm Biopharm. 2001;52(2):181–90. https://doi.org/10.1016/S0939-6411(01)00162-X.
Reitz C, Kleinebudde P. Solid lipid extrusion of sustained release dosage forms. Eur J Pharm Biopharm. 2007;67(2):440–8. https://doi.org/10.1016/j.ejpb.2007.03.008.
Hasa D, Perissutti B, Grassi M, Zacchigna M, Pagotto M, Lenaz D, et al. Melt extruded helical waxy matrices as a new sustained drug delivery system. Eur J Pharm Biopharm. 2011;79(3):592–600. https://doi.org/10.1016/j.ejpb.2011.07.012.
Roblegg E, Jager E, Hodzic A, Koscher G, Mohr S, Zimmer A, et al. Development of sustained-release lipophilic calcium stearate pellets via hot melt extrusion. Eur J Pharm Biopharm. 2011;79(3):635–45. https://doi.org/10.1016/j.ejpb.2011.07.004.
Sax G, Winter G. Mechanistic studies on the release of lysozyme from twin-screw extruded lipid implants. J Control Release. 2012;163(2):187–94. https://doi.org/10.1016/j.jconrel.2012.08.025.
Tan DC, Chin WW, Tan EH, Hong S, Gu W, Gokhale R. Effect of binders on the release rates of direct molded verapamil tablets using twin-screw extruder in melt granulation. Int J Pharm. 2014;463(1):89–97. https://doi.org/10.1016/j.ijpharm.2013.12.053.
Vithani K, Cuppok Y, Mostafa S, Slipper IJ, Snowden MJ, Douroumis D. Diclofenac sodium sustained release hot melt extruded lipid matrices. Pharm Dev Technol. 2014;19(5):531–8. https://doi.org/10.3109/10837450.2013.805775.
Patil H, Tiwari RV, Upadhye SB, Vladyka RS, Repka MA. Formulation and development of pH-independent/dependent sustained release matrix tablets of ondansetron HCl by a continuous twin-screw melt granulation process. Int J Pharm. 2015;496(1):33–41. https://doi.org/10.1016/j.ijpharm.2015.04.009.
Laukamp EJ, Vynckier AK, Voorspoels J, Thommes M, Breitkreutz J. Development of sustained and dual drug release co-extrusion formulations for individual dosing. Eur J Pharm Biopharm. 2015;89:357–64. https://doi.org/10.1016/j.ejpb.2014.12.027.
Monteyne T, Adriaensens P, Brouckaert D, Remon JP, Vervaet C, De Beer T. Stearic acid and high molecular weight PEO as matrix for the highly water soluble metoprolol tartrate in continuous twin-screw melt granulation. Int J Pharm. 2016;512(1):158–67. https://doi.org/10.1016/j.ijpharm.2016.07.035.
Vaingankar P, Amin P. Continuous melt granulation to develop high drug loaded sustained release tablet of metformin HCl. Asian J Pharm Sci. 2017;12(1):37–50. https://doi.org/10.1016/j.ajps.2016.08.005.
Vithani K, Maniruzzaman M, Slipper IJ, Mostafa S, Miolane C, Cuppok Y, et al. Sustained release solid lipid matrices processed by hot-melt extrusion (HME). Colloids Surf B Biointerfaces. 2013;110:403–10. https://doi.org/10.1016/j.colsurfb.2013.03.060.
Nart V, Beringhs AO, Franca MT, de Espindola B, Pezzini BR, Stulzer HK. Carnauba wax as a promising excipient in melt granulation targeting the preparation of mini-tablets for sustained release of highly soluble drugs. Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 1):250–7. https://doi.org/10.1016/j.msec.2016.07.070.
Vynckier AK, Lin H, Zeitler JA, Willart JF, Bongaers E, Voorspoels J, et al. Calendering as a direct shaping tool for the continuous production of fixed-dose combination products via co-extrusion. Eur J Pharm Biopharm. 2015;96:125–31. https://doi.org/10.1016/j.ejpb.2015.07.023.
Vanhoorne V, Vanbillemont B, Vercruysse J, De Leersnyder F, Gomes P, Beer TD, et al. Development of a controlled release formulation by continuous twin screw granulation: influence of process and formulation parameters. Int J Pharm. 2016;505(1–2):61–8. https://doi.org/10.1016/j.ijpharm.2016.03.058.
Shergill M, Patel M, Khan S, Bashir A, McConville C. Development and characterisation of sustained release solid dispersion oral tablets containing the poorly water soluble drug disulfiram. Int J Pharm. 2016;497(1–2):3–11. https://doi.org/10.1016/j.ijpharm.2015.11.029.
Zhang F, McGinity JW. Properties of hot-melt extruded theophylline tablets containing poly(vinyl acetate). Drug Dev Ind Pharm. 2000;26(9):931–42. https://doi.org/10.1081/DDC-100101320.
Sarraf AG, Cherkaoui S, Jordan O, Gurny R, Doelker E. Controlled drug release from melt-extrudates through processing parameters: a chemometric approach. Int J Pharm. 2015;481(1–2):9–17. https://doi.org/10.1016/j.ijpharm.2014.12.046.
Zhu Y, Shah NH, Malick AW, Infeld MH, McGinity JW. Controlled release of a poorly water-soluble drug from hot-melt extrudates containing acrylic polymers. Drug Dev Ind Pharm. 2006;32(5):569–83. https://doi.org/10.1080/03639040500528996.
Quinten T, Andrews GP, De Beer T, Saerens L, Bouquet W, Jones DS, et al. Preparation and evaluation of sustained-release matrix tablets based on metoprolol and an acrylic carrier using injection moulding. AAPS PharmSciTech. 2012;13(4):1197–211. https://doi.org/10.1208/s12249-012-9848-6.
Bouman J, Belton P, Venema P, van der Linden E, de Vries R, Qi S. Controlled release from zein matrices: interplay of drug hydrophobicity and pH. Pharm Res. 2016;33(3):673–85. https://doi.org/10.1007/s11095-015-1818-8.
Zhang F. Physicochemical properties and mechanisms of drug release from melt-extruded granules consisting of chlorpheniramine maleate and Eudragit FS. Drug Dev Ind Pharm. 2016;42(4):563–71. https://doi.org/10.3109/03639045.2015.1054832.
Ravina-Eirin E, Sanchez-Rodriguez B, Gomez-Amoza JL, Martinez-Pacheco R. Evaluation of the hyperbranched polymer Hybrane H1500 for production of matricial controlled-release particles by hot-melt extrusion. Int J Pharm. 2014;461(1–2):469–77. https://doi.org/10.1016/j.ijpharm.2013.12.019.
Henrist D, Lefebvre RA, Remon JP. Bioavailability of starch based hot stage extrusion formulations. Int J Pharm. 1999;187(2):185–91. https://doi.org/10.1016/S0378-5173(99)00186-6.
Vaz CM, van Doeveren P, Reis RL, Cunha AM. Soy matrix drug delivery systems obtained by melt-processing techniques. Biomacromolecules. 2003;4(6):1520–9. https://doi.org/10.1021/bm034050i.
Bouman J, Belton P, Venema P, van der Linden E, de Vries R, Qi S. The development of direct extrusion-injection moulded zein matrices as novel oral controlled drug delivery systems. Pharm Res. 2015;32(8):2775–86. https://doi.org/10.1007/s11095-015-1663-9.
Ma DC, Djemai A, Gendron CM, Xi HM, Smith M, Kogan J, et al. Development of a HPMC-based controlled release formulation with hot melt extrusion (HME). Drug Dev Ind Pharm. 2013;39(7):1070–83. https://doi.org/10.3109/03639045.2012.702350.
Liu X, Lu M, Guo Z, Huang L, Feng X, Wu C. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm Res. 2012;29(3):806–17. https://doi.org/10.1007/s11095-011-0605-4.
Almeida A, Brabant L, Siepmann F, De Beer T, Bouquet W, Van Hoorebeke L, et al. Sustained release from hot-melt extruded matrices based on ethylene vinyl acetate and polyethylene oxide. Eur J Pharm Biopharm. 2012;82(3):526–33. https://doi.org/10.1016/j.ejpb.2012.08.008.
Quinten T, De Beer T, Onofre FO, Mendez-Montealvo G, Wang YJ, Remon JP, et al. Sustained-release and swelling characteristics of xanthan gum/ethylcellulose-based injection moulded matrix tablets: in vitro and in vivo evaluation. J Pharm Sci. 2011;100(7):2858–70. https://doi.org/10.1002/jps.22480.
Quinten T, Gonnissen Y, Adriaens E, De Beer T, Cnudde V, Masschaele B, et al. Development of injection moulded matrix tablets based on mixtures of ethylcellulose and low-substituted hydroxypropylcellulose. Eur J Pharm Sci. 2009;37(3–4):207–16. https://doi.org/10.1016/j.ejps.2009.02.006.
Feng X, Vo A, Patil H, Tiwari RV, Alshetaili AS, Pimparade MB, et al. The effects of polymer carrier, hot melt extrusion process and downstream processing parameters on the moisture sorption properties of amorphous solid dispersions. J Pharm Pharmacol. 2016;68(5):692–704. https://doi.org/10.1111/jphp.12488.
Crowley MM, Schroeder B, Fredersdorf A, Obara S, Talarico M, Kucera S, et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int J Pharm. 2004;269(2):509–22. https://doi.org/10.1016/j.ijpharm.2003.09.037.
Feng X, Ye X, Park J-B, Lu W, Morott J, Beissner B, et al. Evaluation of the recrystallization kinetics of hot-melt extruded polymeric solid dispersions using an improved Avrami equation. Drug Dev Ind Pharm. 2015;41(9):1479–87. https://doi.org/10.3109/03639045.2014.958755.
Keen JM, Hughey JR, Bennett RC, Jannin V, Rosiaux Y, Marchaud D, et al. Effect of tablet structure on controlled release from supersaturating solid dispersions containing glyceryl behenate. Mol Pharm. 2015;12(1):120–6. https://doi.org/10.1021/mp500480y.
Vo AQ, Feng X, Morott JT, Pimparade MB, Tiwari RV, Zhang F, et al. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur J Pharm Biopharm. 2016;98:108–21. https://doi.org/10.1016/j.ejpb.2015.11.015.
Vo AQ, Feng X, Pimparade M, Ye X, Kim DW, Martin ST, et al. Dual-mechanism gastroretentive drug delivery system loaded with an amorphous solid dispersion prepared by hot-melt extrusion. Eur J Pharm Sci. 2017;102:71–84. https://doi.org/10.1016/j.ejps.2017.02.040.
Fukuda M, Peppas NA, McGinity JW. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J Control Release. 2006;115(2):121–9. https://doi.org/10.1016/j.jconrel.2006.07.018.
Nakamichi K, Yasuura H, Fukui H, Oka M, Izumi S. Evaluation of a floating dosage form of nicardipine hydrochloride and hydroxypropylmethylcellulose acetate succinate prepared using a twin-screw extruder. Int J Pharm. 2001;218(1–2):103–12. https://doi.org/10.1016/S0378-5173(01)00617-2.
Liu X, Feng X, Williams RO, Zhang F. Characterization of amorphous solid dispersions. J Pharm Investig. 2017:1–23.
Liu X, Zhou L, Zhang F. Reactive melt extrusion to improve the dissolution performance and physical stability of naproxen amorphous solid dispersions. Mol Pharm. 2017;14(3):658–73. https://doi.org/10.1021/acs.molpharmaceut.6b00960.
Quinten T, De Beer T, Almeida A, Vlassenbroeck J, Van Hoorebeke L, Remon JP, et al. Development and evaluation of injection-molded sustained-release tablets containing ethylcellulose and polyethylene oxide. Drug Dev Ind Pharm. 2011;37(2):149–59. https://doi.org/10.3109/03639045.2010.498426.
Roth W, Setnik B, Zietsch M, Burst A, Breitenbach J, Sellers E, et al. Ethanol effects on drug release from verapamil Meltrex (R), an innovative melt extruded formulation. Int J Pharm. 2009;368(1–2):72–5. https://doi.org/10.1016/j.ijpharm.2008.09.052.
Kipping T, Rein HA. New method for the continuous production of single dosed controlled release matrix systems based on hot-melt extruded starch: analysis of relevant process parameters and implementation of an in-process control. Eur J Pharm Biopharm. 2013;84(1):156–71. https://doi.org/10.1016/j.ejpb.2012.12.013.
Vynckier AK, Dierickx L, Saerens L, Voorspoels J, Gonnissen Y, De Beer T, et al. Hot-melt co-extrusion for the production of fixed-dose combination products with a controlled release ethylcellulose matrix core. Int J Pharm. 2014;464(1–2):65–74. https://doi.org/10.1016/j.ijpharm.2014.01.028.
Zema L, Melocchi A, Maroni A, Gazzaniga A. Three-dimensional printing of medicinal products and the challenge of personalized therapy. J Pharm Sci. 2017;106(7):1697–705. https://doi.org/10.1016/j.xphs.2017.03.021.
Crump SS. Apparatus and method for creating three-dimensional objects. Google Patents; 1992.
O’Connor TF, LX Y, Lee SL. Emerging technology: a key enabler for modernizing pharmaceutical manufacturing and advancing product quality. Int J Pharm. 2016;509(1–2):492–8. https://doi.org/10.1016/j.ijpharm.2016.05.058.
Patil H, Feng X, Ye X, Majumdar S, Repka MA. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach. AAPS J. 2015;17(1):194–205. https://doi.org/10.1208/s12248-014-9674-8.
Goyanes A, Robles Martinez P, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–63. https://doi.org/10.1016/j.ijpharm.2015.04.069.
Zhang J, Feng X, Patil H, Tiwari RV, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519(1–2):186–97. https://doi.org/10.1016/j.ijpharm.2016.12.049.
Goyanes A, Chang H, Sedough D, Hatton GB, Wang J, Buanz A, et al. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int J Pharm. 2015;496(2):414–20. https://doi.org/10.1016/j.ijpharm.2015.10.039.
Goyanes A, Wang J, Buanz A, Martinez-Pacheco R, Telford R, Gaisford S, et al. 3D printing of medicines: engineering novel oral devices with unique design and drug release characteristics. Mol Pharm. 2015;12(11):4077–84. https://doi.org/10.1021/acs.molpharmaceut.5b00510.
Zhang J, Yang W, Vo AQ, Feng X, Ye X, Kim DW, et al. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: structure and drug release correlation. Carbohydr Polym. 2017;177:49–57. https://doi.org/10.1016/j.carbpol.2017.08.058.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3(3):1377–97. https://doi.org/10.3390/polym3031377.
Wang Y, Wen Q, Choi S. FDA’s regulatory science program for generic PLA/PLGA-based drug products. Am Pharm Rev. 2016;19(4):5–9.
Spada LT, Ghebremeskel AN, Robinson MR. Biodegradable alpha-2 agonist polymeric implants and therapeutic uses thereof. Google Patents; 2015.
Ghebremeskel AN, Robinson MR. Prostamide-containing intraocular implants and methods of use thereof. Google Patents; 2016.
Shiah JG, Bhagat R, Blanda WM, Nivaggioli T, Peng L, Chou D, et al. Ocular implant made by a double extrusion process US8034370. Google Patents; 2011.
Shiah JG, Bhagat R, Blanda WM, Nivaggioli T, Peng L, Chou D, et al. Ocular implant made by a double extrusion process US8034366. Google Patents; 2011.
Helbling IM, Ibarra JC, Luna JA. The optimization of an intravaginal ring releasing progesterone using a mathematical model. Pharm Res. 2014;31(3):795–808. https://doi.org/10.1007/s11095-013-1201-6.
Schneider C, Langer R, Loveday D, Hair D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J Control Release. 2017;262:284–95. https://doi.org/10.1016/j.jconrel.2017.08.004.
Kleiner LW, Wright JC, Wang Y. Evolution of implantable and insertable drug delivery systems. J Control Release. 2014;181:1–10. https://doi.org/10.1016/j.jconrel.2014.02.006.
JAHV L, MAB K, Vromans H. In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. Int J Pharm. 2002;232:163–73.
JAHV L, MAB K, Vromans H. Effect of supersaturation and crystallization phenomena on the release properties of a controlled release device based on EVA copolymer. J Control Release. 2002;82:309–19.
Genina N, Holländer J, Jukarainen H, Mäkilä E, Salonen J, Sandler N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur J Pharm Sci. 2016;90:53–63. https://doi.org/10.1016/j.ejps.2015.11.005.
Costantini LC. The development of ProNeura technology for the treatment of addictions. In: Dean RL, Bilsky EJ, Negus SS, editors. Opiate receptors and antagonists: from bench to clinic. Totowa: Humana Press; 2009. p. 689–708. https://doi.org/10.1007/978-1-59745-197-0_37.
U.S. Food and Drug Administration. FDA approves first buprenorphine implant for treatment of opioid dependence. 2016.
Patel R, Bucalo L. Implantable polymeric device for sustained release of buprenorphine. Google Patents; 2004.
Patel RA, Bucalo LR. Implantable polymeric device for sustained release of buprenorphine with minimal initial burst. Google Patents; 2008.
Patel R. Methods and device for treating opioid addiction. Google Patents; 2014.
Bourges J, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, et al. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58(11):1182–202. https://doi.org/10.1016/j.addr.2006.07.026.
Carcaboso ÁM, Chiappetta DA, Höcht C, Blake MG, Boccia MM, Baratti CM, et al. In vitro/in vivo characterization of melt-molded gabapentin-loaded poly (epsilon-caprolactone) implants for sustained release in animal studies. Eur J Pharm Biopharm. 2008;70(2):666–73. https://doi.org/10.1016/j.ejpb.2008.05.031.
Chen W, Wu Z, Yang H, Guo S, Li D, Cheng L. In vitro and in vivo evaluation of injectable implants for intratumoral delivery of 5-fluorouracil. Pharm Dev Technol. 2014;19(2):223–31. https://doi.org/10.3109/10837450.2013.769568.
Clark MR, Johnson TJ, Mccabe RT, Clark JT, Tuitupou A, Elgendy H, et al. A hot-melt extruded intravaginal ring for the sustained delivery of the antiretroviral microbicide UC781. J Pharm Sci. 2012;101(2):576–87. https://doi.org/10.1002/jps.22781.
Ward RS, Wang S, Li L, Chalasani D, Kiser P, Clark MR. Intravaginal ring comprising polyurethane composition for drug delivery. Google Patents; 2016.
Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J Control Release. 2007;122(3):338–44. https://doi.org/10.1016/j.jconrel.2007.05.034.
Sivalingam G, Vijayalakshmi S, Madras G. Enzymatic and thermal degradation of poly (ε-caprolactone), poly (D, L-lactide), and their blends. Ind Eng Chem Res. 2004;43(24):7702–9. https://doi.org/10.1021/ie049589r.
Kreye F, Siepmann F, Siepmann J. Lipid implants as drug delivery systems. Expert Opin Drug Deliv. 2008;5(3):291–307. https://doi.org/10.1517/17425247.5.3.291.
Kreye F, Siepmann F, Willart J, Descamps M, Siepmann J. Drug release mechanisms of cast lipid implants. Eur J Pharm Biopharm. 2011;78(3):394–400. https://doi.org/10.1016/j.ejpb.2011.02.011.
Kreye F, Siepmann F, Zimmer A, Willart JF, Descamps M, Siepmann J. Cast lipid implants for controlled drug delivery: importance of the tempering conditions. J Pharm Sci. 2011;100(8):3471–81. https://doi.org/10.1002/jps.22574.
Even MP, Young K, Winter G, Hook S, Engert J. In vivo investigation of twin-screw extruded lipid implants for vaccine delivery. Eur J Pharm Biopharm. 2014;87(2):338–46. https://doi.org/10.1016/j.ejpb.2014.02.014.
Even MP, Bobbala S, Kooi KL, Hook S, Winter G, Engert J. Impact of implant composition of twin-screw extruded lipid implants on the release behavior. Int J Pharm. 2015;493(1–2):102–10. https://doi.org/10.1016/j.ijpharm.2015.06.052.
Even M-P, Bobbala S, Gibson B, Hook S, Winter G, Engert J. Twin-screw extruded lipid implants containing TRP2 peptide for tumour therapy. Eur J Pharm Biopharm. 2017;114:79–87. https://doi.org/10.1016/j.ejpb.2016.12.033.
Vollrath M, Engert J, Winter G. Long-term release and stability of pharmaceutical proteins delivered from solid lipid implants. Eur J Pharm Biopharm. 2017;117:244–55. https://doi.org/10.1016/j.ejpb.2017.04.017.
Hagen TA, Lo JB, Thombre AG, Herbig SM, Appel LE, Crew MD, et al., inventors; Pfizer, Inc., assignee. Azithromycin dosage forms with reduced side effect. US patent 6,984,403. 2006.
Ray RJ, Appel LE, Friesen DT, Crew MD, Schockey JR, inventors; Pfizer, Inc., assignee. Multiparticulate compositions with improved stability. US patent 7,736,672. 2010.
Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse, and treatment in the United States: an update. Am J Psychiatry. 2016;173(1):18–26. https://doi.org/10.1176/appi.ajp.2015.15020262.
Bartholomaeus JH, Schwier S, Brett M, Stahlberg H-J, Galia E, Strothmann K. New abuse deterrent formulation technology for immediate-release opioids. Drug Dev Deliv. 2013;13(8):76–81.
Rahman Z, Yang Y, Korang-Yeboah M, Siddiqui A, Xu X, Ashraf M, et al. Assessing impact of formulation and process variables on in-vitro performance of directly compressed abuse deterrent formulations. Int J Pharm. 2016;502(1):138–50. https://doi.org/10.1016/j.ijpharm.2016.02.029.
Bartholomaeus JH, Arkenau-Maric E, Galia E. Opioid extended-release tablets with improved tamper-resistant properties. Expert Opin Drug Deliv. 2012;9(8):879–91. https://doi.org/10.1517/17425247.2012.698606.
Oshlack B, Chasin M, Huang H-P, inventors; Euro-Celtique, S.A., assignee. Extruded orally administrable opioid formulations. US patent 5,958,452. 1999.
Walden M, Nicholls FA, Smith KJ, Tucker GT. The effect of ethanol on the release of opioids from oral prolonged-release preparations. Drug Dev Ind Pharm. 2007;33(10):1101–11. https://doi.org/10.1080/03639040701377292.
Groenewegen RJJ. Drug delivery system for two or more active substances. Google Patents; 1999.
Hv L, Veurink J, Kruft M-A, Vromans H. Influence of spinline stress on release properties of a coaxial controlled release device based on EVA polymers. Pharm Res. 2004;21(10):1811–7.
Loreti G, Maroni A, Del Curto MD, Melocchi A, Gazzaniga A, Zema L. Evaluation of hot-melt extrusion technique in the preparation of HPC matrices for prolonged release. Eur J Pharm Sci. 2014;52:77–85. https://doi.org/10.1016/j.ejps.2013.10.014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Feng, X., Zhang, F. Twin-screw extrusion of sustained-release oral dosage forms and medical implants. Drug Deliv. and Transl. Res. 8, 1694–1713 (2018). https://doi.org/10.1007/s13346-017-0461-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0461-9