Abstract
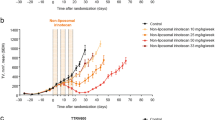
Contemporary chemotherapy is limited by disseminated, resistant cancer. Targeting nanoparticulate drug delivery systems that encapsulate synergistic drug combinations are a rational means to increase the therapeutic index of chemotherapeutics. A lipopolymeric micelle co-encapsulating an in vitro optimized, synergistic fixed-ratio combination of paclitaxel (PTX) and clofazimine (B663) has been developed and called Riminocelles™. The present pre-clinical study investigated the acute toxicity, systemic exposure, repeat dose toxicity and efficacy of Riminocelles in parallel to Taxol® at an equivalent PTX dose of 10 mg/kg. Daily and weekly dosing schedules were evaluated against Pgp-expressing human colon adenocarcinoma (HCT-15) xenografts implanted subcutaneously in athymic mice. Riminocelles produced statistically significant (p < 0.05) tumor growth delays of 3.2 and 2.7 days for the respective schedules in contrast to Taxol delaying growth by 0.5 and 0.6 days. Using the control tumor doubling time of 4.2 days, tumor-cell-kill values of 0.23 for Riminocelles and 0.04 for Taxol following daily schedules were calculated. A significant weight loss of 5.7 % after 14 days (p < 0.05) relative to the control group (n = 8) was observed for the daily Taxol group whereas Riminocelles did not incur significant weight loss neither were blood markers of toxicity elevated after acute administration (n = 3). The safety and efficacy of Riminocelles is statistically superior to Taxol. However, passive tumor targeting was not achieved and the tumor burden progressed quickly. Prior to further animal studies, the in vivo thermodynamic instability of the simple lipopolymeric micellular delivery system requires improvement so as to maintain and selectively deliver the fixed-ratio drug combination.






Similar content being viewed by others
Abbreviations
- MDR:
-
Multi-drug resistance
- FRDC:
-
Fixed-ratio drug combination
- NDDS:
-
Nanoparticulate drug delivery system
- Paclitaxel:
-
PTX
- Clofazimine:
-
B663
References
Hennenfent KL, Govindan R. Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol. 2006;17(5):735–49.
Ma P, Mumper R. Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol. 2013;4(2):1000164.
Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: molecular mechanism and pharmacologic manipulation. Curr Cancer Drug Targets. 2003;3(1):1–19.
Goldie JH. Drug resistance in cancer: a perspective. Cancer Metast Rev. 2001;20(1–2):63–8.
Brózik A, Hegedüs C, Erdei Z, Hegedűs T, Özvegy-Laczka C, Szakács G, et al. Tyrosine kinase inhibitors as modulators of ATP binding cassette multidrug transporters: substrates, chemosensitizers or inducers of acquired multidrug resistance? Expert Opin Drug Met. 2011;7(5):623–42.
van de Vrie W, Marquet RL, Stoter G, De Bruijn EA, Eggermont MM. In vivo model systems in P-glycoprotein-mediated multidrug resistance. Crit Rev Clin Lab. 1998;35:1–57.
Callaghan R, Luk F, Bebawy M. Inhibition of the multidrug resistance p-glycoprotein: time for a change of strategy? Drug Metab Dispos. 2014;42(4):623–31.
Frei E, Elias A, Wheeler C, Richardson P, Hryniuk W. The relationship between high-dose treatment and combination chemotherapy: the concept of summation dose intensity. Clin Cancer Res. 1998;4:2027–37.
Ramsay E, Dos Santos N, Dragowska W, Laskin J, Bally M. The formulation of lipid-based nanotechnologies for the delivery of fixed dose anticancer drug combinations. Curr Drug Deliv. 2005;2:341–51.
Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–63.
Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7(4):216–23.
Zimmerman GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12(1–2):34–42.
Chiu G, Wong M, Ling L, Shaikh I, Tan K, Chaudhury A, et al. Lipid-based nanoparticulate systems for the delivery of anti-cancer drug cocktails: implications on pharmacokinetics and drug toxicities. Curr Drug Metab. 2009;10(8):861–74.
Van Rensburg CEJ, Van Staden AM, Anderson R. The Riminophenazine agents Clofazimine and B669 inhibit the proliferation of cancer cell lines in vitro by phospholipase A2-mediated oxidative and nonoxidative mechanism. Cancer Res. 1993;53(2):318–23.
Van Rensburg C, Theron A, Chasen P. The riminophenazine agents clofazimine and B669 inhibit the proliferation of intrinsically multidrug resistant carcinoma cell lines. Oncol Rep. 1996;3(1):103–6.
Van Niekerk E, Sullivan JF, Joone GK, van Rensburg CEJ. Tetramethylpiperidine-substituted phenazines inhibit the proliferation of intrinsically resistant carcinoma cell lines. Invest New Drugs. 2001;19(3):211–7.
Sri-Pathmanathan R, Plumb J, Fearon K. Clofazimine alters the energy metabolism and inhibits the growth rate of a human lung-cancer cell line in vitro and in vivo. Int J Cancer. 1994;56(6):900–5.
Van Rensburg CEJ, Anderson R, Myer MS, Joone GK, O’Sullivan JF. The riminophenazine agents clofazimine agents clofazimine and B669 reverse acquired multidrug resistance in a human lung cancer cell line. Cancer Lett. 1994;85(1):59–63.
Myer MS, Van Rensburg CEJ. Chemosensitizing interactions of clofazimine and B669 with human K562 erythroleukaemia cells with varying levels of expression of P-glycoprotein. Cancer Lett. 1996;99(1):73–8.
Van Rensburg CEJ, Anderson R, Joone G, Myer MS, O’Sullivan JF. Novel tetramethylpiperidine-substituted phenazine are potent inhibitors of P-glycoprotein activity in a multidrug resistant cancer cell line. Anticancer Drugs. 1997;8(7):708–13.
Van Rensburg CEJ, Joone GK, O’Sullivan JF. Clofazimine and B4121 sensitize an intrinsically resistant human colon cancer cell line to P-glycoprotein substrates. Oncol Rep. 2000;7(1):193–5.
Van Rensburg CEJ, Anderson R, O’Sullivan JF. Riminophenazine compounds: pharmacology and antineoplastic. Crit Rev Oncol Hematol. 1997;25(1):55–67.
Rhodes PM, Wilkie D. Antimitochondrial activity of Lamprene in Saccharomyce cerevisiae. Biochem Pharmacol. 1973;22:1047–56.
Morrison NE, Marley GM. Clofazimine binding studies with deoxyribonucleic acid. Int J Lepr Other Mycobact Dis. 1976;44(4):475–81.
Van Rensburg C, Durandt C, Garlinski P, O’Sullivan J. Evaluation of the antineoplastic activities of the riminophenazine agents clofazimine and B669 in tumor-bearing rats and mice. Int J Oncol. 1993;3(5):1011–3.
Ruff P, Chasen M, Long J, Van Rensburg C. A phase II study of oral clofazimine in unresectable and metastatic hepatocellular carcinoma. Ann Oncol. 1998;9(2):217–9.
Falkson C, Falkson G. A phase II evaluation of clofazimine plus doxorubicin in advanced, unresectable primary hepatocellular carcinoma. Oncology. 1999;57(3):232–5.
Gao Z, Lukyanov A, Singhal A, Torchilin V. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2(9):979–82.
Lukyanov A, Torchilin V. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56(9):1273–89.
Torchilin V. Lipid-core micelles for targeted drug delivery. Curr Drug Deliv. 2005;2(4):319–27.
Kastantin M, Missirlis D, Black M, Ananthanarayanan B, Peters D, Tirrell M. Thermodynamic and kinetic instability of DSPE-PEG (2000) micelles in the presence of bovine serum albumin. J Phys Chem B. 2010;114(39):12632–40.
Uchiyama-Kokubu N, Watanabe T, Cohen D. Intracellular levels of two cyclosporine derivatives valspodar (PSC 833) and cyclosporin a closely associated with multidrug resistance-modulating activity in sublines of human colorectal adenocarcinoma HCT-15. Jpn J Cancer Res. 2001;92(10):1116–26.
Plowman J, Dykes D, Hollingshead M, Simpson-Herren L, Alley M. Human tumor xenograft models in NCI drug development. In: Teicher BA, editor. Anticancer drug development guide. New Jersey: Humana Press; 1997. p. 101–26.
Teicher BA. In vivo tumor response endpoints. In: Teicher BA, editor. Tumor models in cancer research. New Jersey: Humana Press; 2002. p. 593–616.
Corbett T, Valeriote F, LoRusso P, Polin L, Panchapor C, Pugh S, et al. In vivo methods for screening and preclinical testing. In: Teicher BA, editor. Anticancer drug development guide. New Jersey: Humana Press; 1997. p. 75–100.
Holdiness MR. Clinical pharmacokinetics of Clofazimine: a review. Clin Pharmacokinet. 1989;16:74–85.
Corbett T, Polin L, Roberts B, Lawson A, Leopold W, White K, et al. Transplantable syngeneic rodent tumors: solid tumors in mice. In: Teicher BA, editor. Tumor models in cancer research. New Jersey: Humana Press; 2002. p. 41–72.
International agency for research on cancer (IARC), World Health Organisation (WHO).GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed June 2014.
Menon K, Teicher BA. Metastasis models. In: Teicher BA, editor. Tumor models in cancer research. New Jersey: Humana Press; 2002. p. 277–92.
Kingston D. Recent advances in the chemistry of taxol. J Nat Prod. 2000;63(5):726–34.
Koziara JM, Whisman TR, Tseng MT, Mumper RJ. In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release. 2006;12:312–9.
Cosco D, Paolino D, Maiuolo J. Liposomes as multicompartmental carriers for multidrug delivery in anticancer chemotherapy. Drug Deliv Transl Res. 2011;1:66–75.
Milane L, Duan Z, Amiji M. Pharmacokinetics and biodistribution of lonidamine/paclitaxel loaded, EGFR-targeted nanoparticles in an orthotopic animal model of multi-drug resistant breast cancer. Nanomed-Nanotechnol. 2011;7:435–44.
Li F, Danquah M, Singh S, Wu H, Mahato R. Paclitaxel- and lapatinib-loaded lipopolymeric micelles overcome multidrug resistance in prostate cancer. Drug Deliv Transl Res. 2011;1:420–8.
Sarisozen C, Vural I, Levchenko T, Hincal A, Torchilin V. Long-circulating PEG-PE micelles co-loaded with paclitaxel and elacridar (GG918) overcome multidrug resistance. Drug Deliv. 2012;19:363–70.
Sarisozen C, Vural I, Levchenko T, Hincal A, Torchilin V. PEG-PE based micelles co-loaded with paclitaxel and cyclosporine A or loaded with paclitaxel and targeted by anticancer antibody overcome drug resistance in cancer cells. Drug Deliv. 2012;19:169–76.
Weissig V, Whiteman KR, Torchilin V. Accumulation of protein-loaded long circulating micelles and liposomes in subcutaneous Lewis lung carcinoma in mice. Pharm Res. 1998;15(10):1552–6.
Lukyanov A, Gao Z, Mazzola L, Torchilin V. Polyethylene glycol-diacyl lipid micelles demonstrate increased accumulation in subcutaneous tumours in mice. Pharm Res. 2002;19(10):1424–9.
Vakil R, Kwon G. Effect of cholesterol on the release of amphotericin B from PEG-phospholipid micelles. Mol Pharm. 2007;5(1):98–104.
Acknowledgments
Financial support for this study was provided by BioPAD (BPH 004) and the Department of Pharmacology, University of Pretoria.
Declaration of ethical standards
The experiments in this work comply with the current laws and standards of the countries in which they were performed.
Conflict of interest
The authors declare no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koot, D., Cromarty, D. Anticancer efficacy and toxicokinetics of a novel paclitaxel-clofazimine nanoparticulate co-formulation. Drug Deliv. and Transl. Res. 5, 257–267 (2015). https://doi.org/10.1007/s13346-015-0222-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0222-6