Abstract
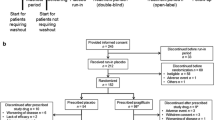
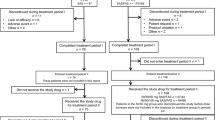
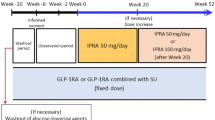
Sulfonylureas are often used alone or in combination with other drugs for treating type 2 diabetes. Ipragliflozin is a sodium/glucose cotransporter 2 inhibitor that enhances urinary glucose excretion and improves glycemic control. We examined the efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese type 2 diabetes patients with inadequate glycemic control in a phase III study. Patients were randomized, double-blind, to 50 mg ipragliflozin or placebo for 24 weeks, followed by a 28-week open-label extension in which all patients received either 50 or 100 mg ipragliflozin. The primary endpoint was the change in HbA1c from baseline to week 24 (last observation carried forward). Overall, 166 patients were prescribed ipragliflozin and 77 were prescribed placebo. The adjusted mean change in HbA1c from baseline to week 24 between the ipragliflozin and placebo groups was −1.14 % (P < 0.001). The reductions in fasting plasma glucose and body weight were significantly greater in the ipragliflozin group (placebo-adjusted mean change: −38.0 mg/dl and −1.32 kg, respectively; both, P < 0.001). These changes were maintained until the end of the open-label extension. There were more treatment-emergent adverse events in the ipragliflozin group than in the placebo group (75.9 vs. 61.8 %; P = 0.032). The most common adverse events with a higher incidence in the ipragliflozin group than in the placebo group were mild pollakiuria and thirst. Ipragliflozin as an add-on to a sulfonylurea significantly improved glycemic control and reduced body weight, with good tolerability, in Japanese type 2 diabetes patients.




Similar content being viewed by others
References
Sabolić I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojević M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol. 2012;302:C1174–88.
Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–7.
Wright EM. Renal Na+-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10–8.
Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95:34–42.
Neumiller JJ, White JR Jr, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs. 2010;70:377–85.
Veltkamp SA, Kadokura T, Krauwinkel WJ, Smulders RA. Effect of ipragliflozin (ASP1941), a novel selective sodium-dependent glucose co-transporter 2 inhibitor, on urinary glucose excretion in healthy subjects. Clin Drug Investig. 2011;31:839–51.
Kadokura T, Saito M, Utsuno A, Kazuta K, Yoshida S, Kawasaki S, Nagase I, Kageyama S. Ipragliflozin (ASP1941), a selective sodium-dependent glucose cotransporter 2 inhibitor, safely stimulates urinary glucose excretion without inducing hypoglycemia in healthy Japanese subjects. Diabetol Int. 2011;2:172–82.
Schwartz SL, Akinlade B, Klasen S, Kowalski D, Zhang W, Wilpshaar W. Safety, pharmacokinetic, and pharmacodynamic profiles of ipragliflozin (ASP1941), a novel and selective inhibitor of sodium-dependent glucose co-transporter 2, in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13:1219–27.
Fonseca VA, Ferrannini E, Wilding JP, Wilpshaar W, Dhanjal P, Ball G, Klasen S. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2013;27:268–73.
Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013. doi:10.1111/jdi.12156.
Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab. 2013;15:403–9.
Arai K, Matoba K, Hirao K, Matsuba I, Takai M, Takeda H, Kanamori A, Yamauchi M, Mori H, Terauchi Y. Present status of sulfonylurea treatment for type 2 diabetes in Japan: second report of a cross-sectional survey of 15,652 patients. Endocr J. 2010;57:499–507.
Pan C, Yang W, Jia W, Weng J, Tian H. Management of Chinese patients with type 2 diabetes, 1998–2006: the Diabcare-China surveys. Curr Med Res Opin. 2009;25:39–45.
Smulders RA, Zhang W, Veltkamp SA, van Dijk J, Krauwinkel WJ, Keirns J, Kadokura T. No pharmacokinetic interaction between ipragliflozin and sitagliptin, pioglitazone, or glimepiride in healthy subjects. Diabetes Obes Metab. 2012;14:937–43.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int. 2012;3:8–10.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Director, Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Guideline for clinical evaluation of oral hypoglycemic agents. Tokyo: Ministry of Health, Labour and Welfare; 2010.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43.
Tan MH, Baksi A, Krahulec B, Kubalski P, Stankiewicz A, Urquhart R, Edwards G, Johns D, GLAL Study Group. Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care. 2005;28:544–50.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79.
Bolinder J, Ljunggren Ö, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–31.
Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928–38.
Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, Zhao X, Moeckel GW, Samuel VT, Whaley JM, Shulman GI, Kibbey RG. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60:890–8.
Acknowledgments
This study was sponsored by Astellas. Medical writing and editorial support were funded by Astellas and provided by Dr. Nicholas D. Smith and ELMCOM™.
Conflict of interest
NA, TS, KK, AU, HO, SY, and EU are employees of Astellas Pharma, Inc., Japan. AK has acted as a consultant for Astellas Pharma, Inc., and has received consulting fees/honoraria from Astellas Pharma, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinicaltrials.gov identifier: NCT01242215.
EMIT: Study to show the efficacy of ipragliflozin in add-on therapy with sulfonylurea in type 2 diabetes mellitus patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Primary Investigators
Primary Investigators
Kazuo Yamagata (Sakajiri Naika Iin), Daishiro Yamada (Jiyugaoka Yamada Clinic of Internal Medicine), Fuminobu Okuguchi (Okuguchi Clinic of Internal Medicine), Takashi Naitou (Saka General Clinic), Hiroaki Seino (Seino Internal Medicine Clinic), Hiroshi Kouno (Jusendo General Hospital), Hirokazu Shoda (Seiwakai Shoda Hospital), Shuichi Fukuda (Wakakusa Clinic), Yoshio Ohashi (Tokyo Ekimae-building Clinic), Madoka Taguchi (Toshiba General Hospital), Mizuki Kaneshiro (Kaneshiro Diabetes Clinic), Koutaro Fujimoto (Fujimoto Clinic), Ikuro Matsuba (Matsuba Clinic), Takaaki Iwai (Sagamino Central Hospital), Akira Yamauchi (Medical Corp. Rikeikai Suruga Clinic), Shizuo Kajiyama (Kajiyama Clinic), Takehiko Kobayashi (Kobayashi Shinryosho), Kiyomitsu Ikeoka (Medical Corp. Ikeoka Clinic), Emi Kose (Sato Hospital), Bunrei Goto (Medical Corp. Gotou Medical Clinic), Kunihiro Ekawa (Saiseikai Wakayama Hospital), Tetsuji Okuno (Nippon Kokan Fukuyama Hospital), Nobuyuki Abe (Abe Diabetes Clinic), Makoto Kunisaki (Kunisaki Makoto Clinic, Medical Corp. Shinaikai), Syuichi Otabe (Otabe Internal Medicine Clinic), Hideaki Jinnouchi (Jinnouchi Hospital), Masahiko Takai (Medical Corp. Takai Internal Medicine Clinic), Tsuguyoshi Asano (Asano Kanamachi Clinic), Kazuo Satake (Fukui General Clinic), Kazunori Yokoyama (Nikko Memorial Hospital), Munenori Okamoto (Sapporo Century Hospital), Tamayuki Koizumi (Medical Corp. Pieta Association Ishikari Hospital), Mitsutoshi Kato (Kato Clinic of Internal Medicine), Osamu Tomonaga (Tomonaga Clinic), and Yasuhiro Hashiguchi (Tenpozan Internal Clinic).
About this article
Cite this article
Kashiwagi, A., Akiyama, N., Shiga, T. et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int 6, 125–138 (2015). https://doi.org/10.1007/s13340-014-0184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-014-0184-9