Abstract
Background and Objectives
The potent, selective P2X3 receptor antagonist eliapixant (BAY 1817080) is under development for conditions characterized by neuronal hypersensitization. As prominent food effects and limited bioavailability in the fasted state were observed with immediate-release eliapixant tablets, a novel formulation was needed. Accordingly, several novel eliapixant formulations were assessed by in vitro and animal studies in a structured way. The most promising of the formulations was then investigated in a phase I study designed to assess its pharmacokinetics, food effect, and bioavailability in healthy volunteers.
Methods
In vitro non-sink dissolution tests were performed with two amorphous solid dispersion (ASD) granule prototypes compared with pure crystalline eliapixant as a surrogate for the immediate-release formulation. Subsequently, the drug exposure of novel eliapixant formulations under fed and fasted conditions in rats and dogs was assessed to confirm improvements in bioavailability versus the suspension-based formulation. A novel Kollidon VA64®-based eliapixant formulation was identified from the preclinical studies and compared with the original tablet formulation in an open-label, partially randomized, threefold, crossover phase I study, in which healthy males received single oral doses (25–400 mg, fasted/fed). Pharmacokinetic parameters, absolute bioavailability (using an intravenous [13C715N]-eliapixant microdose), relative bioavailability (novel versus original formulation), effect of food, and adverse events (AEs) were evaluated.
Results
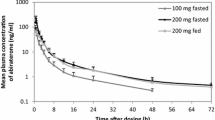
The non-sink dissolution test demonstrated that the two ASD formulations had an improved dissolution rate compared with pure crystalline eliapixant, with a Kollidon VA64-based prototype having the highest dissolution rate. Further testing of this prototype in animal studies confirmed an approximately twofold higher bioavailability compared with the suspension-based formulation. In the phase I study, 30 subjects were randomized. With the novel Kollidon VA64® formulation (400 mg; fasted), area under the concentration–time curve (AUC) and maximum plasma concentration (Cmax) were up to 3.1-fold and 1.7-fold higher, respectively, than with the original formulation (fed). AUC increased dose proportionally between 25 and 100 mg, and less than dose proportionally from 100 to 400 mg. Food had no clinically relevant effect on the novel formulation, with AUC increasing 1.3-fold and Cmax 2.1–2.4-fold (time to maximum concentration was delayed by 1.5–2.25 h). Absolute bioavailability with the novel formulation (100 mg) was 50%. AEs occurred in 57% of patients; most were mild in severity.
Conclusions
The novel eliapixant formulation substantially improved bioavailability compared with immediate-release eliapixant and may be administered with/without food.
Clinical Trial Registration
Clinicaltrials.gov: NCT03773068 (initial registration: 12 December 2018).






Similar content being viewed by others
References
Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99(1):16–34.
Burnstock G. Purine and purinergic receptors. Brain Neurosci Adv. 2018;2:2398212818817494.
North RA. P2X receptors. Philos Trans R Soc Lond B Biol Sci. 2016;371(1700):20150427.
North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–80.
Bonvini SJ, Belvisi MG. Cough and airway disease: the role of ion channels. Pulm Pharmacol Ther. 2017;47:21–8.
Bernier LP, Ase AR, Seguela P. P2X receptor channels in chronic pain pathways. Br J Pharmacol. 2018;175(12):2219–30.
Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;5:69.
Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567(Pt 2):621–39.
Ding S, Yu Q, Wang J, Zhu L, Li T, Guo X, et al. Activation of ATF3/AP-1 signaling pathway is required for P2X3-induced endometriosis pain. Hum Reprod. 2020;35(5):1130–44.
Fabbretti E. ATP P2X3 receptors and neuronal sensitization. Front Cell Neurosci. 2013;7:236.
Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8(Suppl 1):3–26.
Song WJ, Morice AH. Cough hypersensitivity syndrome: a few more steps forward. Allergy Asthma Immunol Res. 2017;9(5):394–402.
Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, et al. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2015;385(9974):1198–205.
McGarvey LP, Birring SS, Morice AH, Dicpinigaitis PV, Pavord ID, Schelfhout J, et al. Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022;399(10328):909–23.
Morice AH, Kitt MM, Ford AP, Tershakovec AM, Wu WC, Brindle K, et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54(1):1900439.
Smith JA, Kitt MM, Butera P, Smith SA, Li Y, Xu ZJ, et al. Gefapixant in two randomised dose-escalation studies in chronic cough. Eur Respir J. 2020;55(3):1901615.
Smith JA, Kitt MM, Morice AH, Birring SS, McGarvey LP, Sher MR, et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med. 2020;8(8):775–85.
Morice AH, Smith JA, McGarvey LP, Birring SS, Parker SM, Turner A, et al. Eliapixant (BAY 1817080), a P2X3 receptor antagonist, in refractory chronic cough: a randomised, placebo-controlled, crossover phase 2a study. Eur Respir J. 2021;58(5):2004240.
Friedrich C, Francke K, Gashaw I, Scheerans C, Klein S, Fels L, et al. Safety and pharmacokinetics of BAY 1817080, a P2X3 receptor antagonist, in healthy subjects: results from a double-blind, randomised study. Brit J Clin Pharmacol. 2021;87:1614–6.
Klein S, Gashaw I, Baumann S, Chang X, Hummel T, Thuß U, et al. First-in-human study of eliapixant (BAY 1817080), a highly selective P2X3 receptor antagonist: tolerability, safety and pharmacokinetics. Br J Clin Pharmacol. 2022;88(10):4552–64.
ClinicalTrials.gov. A 2-part trial to learn more about how BAY1817080 works, how safe it is, and what the right dose is for participants with diabetic neuropathic pain. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04641273. Accessed October 4, 2021.
ClinicalTrials.gov. Clinical study to evaluate the treatment effect and safety of BAY1817080 in patients with overactive bladder (OAB) (OVADER). 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04545580. Accessed October 4, 2021.
ClinicialTrials.gov. Study to gather information how well three different doses of BAY1817080 given twice daily over 12 weeks work in comparison to an inactive pill (placebo) and elagolix in women suffering from pain related to a condition where the tissue that usually grows inside the womb grows outside of the womb (SCHUMANN). 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04614246. Accessed October 4, 2021.
Lui CY, Amidon GL, Berardi RR, Fleisher D, Youngberg C, Dressman JB. Comparison of gastrointestinal pH in dogs and humans: implications on the use of the beagle dog as a model for oral absorption in humans. J Pharm Sci. 1986;75(3):271–4.
McConnell EL, Basit AW, Murdan S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J Pharm Pharmacol. 2008;60(1):63–70.
Acknowledgements
Medical writing services provided by Kristen Brown, PhD, of Adelphi Communications Ltd, Macclesfield, UK were funded by Bayer AG, Berlin, Germany in accordance with Good Publication Practice (GPP3) guidelines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Klaus Francke, Niladri Chattopadhyay, Stefan Klein, Antje Rottmann, Dennis Krickau, and Christian Friedrich are employees of Bayer AG, Berlin, Germany. Jereon van de Wetering declares that he has no conflict of interest.
Ethics approval
The protocol and all protocol amendments were reviewed and approved by the study site’s Independent Ethics Committee/Institutional Review Board before the start of the study. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Council for Harmonization guideline on Good Clinical Practice.
Consent to participate
All subjects provided written, informed consent.
Consent for publication
Not applicable.
Funding
This study was funded by Bayer AG. The funder was responsible for study design, data collection, data analysis, data interpretation, and study report writing. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit this paper for publication.
Data availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations and Pharmaceutical Research and Manufacturers of America principles for responsible clinical trial data sharing, pertaining to scope, time point, and process of data access. Bayer commits to sharing upon request from qualified scientific and medical researchers, patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union as necessary for doing legitimate research. This commitment applies to data on new medicines and indications that have been approved by the European Union and US regulatory agencies on or after January 1, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to do further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Code availability
Not applicable.
Authors’ contributions
K.F., N.C., S.K., A.R., D.K., and C.F. participated in the conception, design, or planning of the studies. K.F., N.C., S.K., A.R., D.K., J.v.d.W., and C.F. acquired and analyzed the data. J.v.d.W. was the principal investigator during the study. K.F., N.C., S.K., A.R., D.K., and C.F. drafted the manuscript. All authors collaborated in the interpretation of study results and review of the manuscript and approved the final draft for submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Francke, K., Chattopadhyay, N., Klein, S. et al. Preclinical and Clinical Pharmacokinetics and Bioavailability in Healthy Volunteers of a Novel Formulation of the Selective P2X3 Receptor Antagonist Eliapixant. Eur J Drug Metab Pharmacokinet 48, 75–87 (2023). https://doi.org/10.1007/s13318-022-00805-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00805-5