Abstract
Purpose
Zytiga (abiraterone acetate, AA) is known to exhibit very low bioavailability and a significant positive food effect in men. The unfavorable pharmacokinetic properties are attributed to the inadequate and variable dissolution of the compound. Using a continuous flow precipitation technology, a novel AA formulation has been developed with improved solubility and dissolution characteristics. The current study was performed to evaluate the pharmacokinetics and safety of this novel formulation in healthy volunteers.
Methods
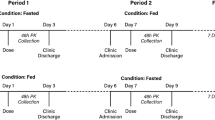
The study was conducted in 11 healthy men aged 47–57 years. All subjects received 3 consecutive single doses of the novel formulation of AA (100 and 200 mg in the fasted state and 200 mg in the fed state). Data were compared with pharmacokinetic and safety data reported for 1000 mg Zytiga, the marketed drug.
Results
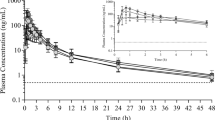
The novel formulation of AA allows rapid absorption of the compound with t max values within 1 hour. Based on AUC values, a ~250 mg dose of the novel formulation is predicted to give the same exposure as 1000 mg Zytiga in the fasted state. The significant positive food effect was also eliminated; actually, a slight, but statistically significant negative food effect was observed. Variability of exposure was significantly reduced when compared to Zytiga. AA administered in the novel formulation was well tolerated with no IMP-related safety AEs reported.
Conclusion
The novel formulation might allow a 75% dose reduction with significant reduction of inter-individual variability. The negative food effect observed requires further investigations; however, elimination of the significant positive food effect could be adequate to negate the restriction of a food label.

Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- C max :
-
Maximal plasma concentration
- t max :
-
Time to maximal plasma concentration
- t 1/2 :
-
Elimination half-life
- SD:
-
Standard deviation
- IMP:
-
Investigational medicinal product
- PiB:
-
Powder in a bottle
- AE:
-
Adverse event
- SAE:
-
Serious adverse event
- TEAE:
-
Treatment emerged adverse event
- CK:
-
Creatine phosphokinase
References
Acharya M, Bernard A, Gonzalez M, Jiao J, De Vries R, Tran N (2012) Open-label, phase I, pharmacokinetic studies of abiraterone acetate in healthy men. Cancer Chemother Pharmacol 69(6):1583–1590
Attard G et al (2008) Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 26(28):4563–4571
EMA/CHMP/542871 (2011) Assessment report for Zytiga procedure no.: EMEA/H/C/002321. Assessment, vol. 44
Chi KN et al (2015) Food effects on abiraterone pharmacokinetics in healthy subjects and patients with metastatic castration-resistant prostate cancer. J Clin Pharmacol 55(12):1406–1414
Geboers S et al (2016) The effect of food on the intraluminal behavior of abiraterone acetate in man. J Pharm Sci 105(9):2974–2981
Li R et al (2012) Abiraterone inhibits 3b-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin Cancer Res 18(13):3571–3579
Matteucci ME, Brettmann BK, Rogers TL, Elder EJ, Williams RO, Johnston KP (2007) Design of potent amorphous drug nanoparticles for rapid generation of highly supersaturated media. Mol Pharm 4(5):782–793
Angi R et al (2014) Novel continuous flow technology for the development of a nanostructured Aprepitant formulation with improved pharmacokinetic properties. Eur J Pharm Biopharm 86(3):361–368
Solymosi T et al (2015) Sirolimus formulation with improved pharmacokinetic properties produced by a continuous flow method. Eur J Pharm Biopharm 94:135–140
Niesz K, Wootsch A, Groualle A, Ötvös Z, Darvas F (2009) Instrument and process for nanoparticles production in continous flow mode. WO 2009/133418 A1
Center for Drug Evaluation and Research (2011) NDA 202-379, Clinical pharmacology and biopharmaceutics review(s). p 20, table 5. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202379Orig1s000ClinPharmR.pdf. Accessed 31 July 2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Solymosi, T., Ötvös, Z., Angi, R. et al. Novel formulation of abiraterone acetate might allow significant dose reduction and eliminates substantial positive food effect. Cancer Chemother Pharmacol 80, 723–728 (2017). https://doi.org/10.1007/s00280-017-3406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3406-6