Abstract
Advances in the understanding of the molecular biology of central nervous system (CNS) tumors prompted a new World Health Organization (WHO) classification scheme in 2021, only 5 years after the prior iteration. The 2016 version was the first to include specific molecular alterations in the diagnoses of a few tumors, but the 2021 system greatly expanded this approach, with over 40 tumor types and subtypes now being defined by their key molecular features. Many tumors have also been reconceptualized into new “supercategories,” including adult-type diffuse gliomas, pediatric-type diffuse low- and high-grade gliomas, and circumscribed astrocytic gliomas. Some entirely new tumors are in this scheme, particularly pediatric tumors. Naturally, these changes will impact how CNS tumor patients are diagnosed and treated, including clinical trial enrollment. This review addresses the most clinically relevant changes in the 2021 WHO book, including diffuse and circumscribed gliomas, ependymomas, embryonal tumors, and meningiomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fifth edition of the WHO Classification of Central Nervous System Tumors was released at the end of 2021, a mere 5 years after the fourth edition was published [1, 2]. Novel techniques such as next generation sequencing, RNA expression profiling, and DNA methylation profiling have paved the way for the discovery and classification of new entities, as well as more precise classification and stratification of existing tumors [3]. These rapid changes in the understanding of the molecular features that define CNS tumors fostered the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) in 2017 to quickly provide updates in the pathological workup of CNS tumors between WHO editions [4]. The fifth edition of the WHO Classification of Central Nervous System Tumors incorporates this updated understanding of the molecular underpinnings of CNS tumors while maintaining their histopathologic roots. The purpose of this review is to discuss how these updates will impact clinical care, focusing on adult-type diffuse gliomas, pediatric-type diffuse gliomas, circumscribed astrocytic gliomas, ependymal tumors, and embryonal tumors (summarized in Table 1), as these are the entities with the most dramatic changes.
Before discussing specific tumors, it is worth mentioning a few general changes in grading. The first is that WHO grades, which were previously listed in Roman numerals, are now listed in Arabic numerals. In addition, grading is now done within tumor types as part of the integrated diagnosis, so although grades still correspond to natural history, there is not necessarily perfect equivalence between the same numerical grade in different types of tumors, i.e., a grade 4 medulloblastoma does not necessarily mean the same prognosis as a grade 4 IDHwt glioblastoma. Also, the term “anaplasia” is no longer employed, instead only “WHO grade 3” is used in the diagnosis. Finally, since a grade 2 glioma (for example) does not necessarily have the same general behavior or prognosis as a grade 2 tumor in WHO classifications of other neoplasms elsewhere in the body, the official usage is “CNS WHO grade 2” not simply “WHO grade 2.” However, for ease of reading, the latter approach is adopted in this review.
Other changes in general nomenclature include “not otherwise specified (NOS)” and “not elsewhere classified (NEC)” [5]. NOS means that the molecular testing required to classify a CNS lesion is not available. For example, if a supratentorial lesion has ependymal morphology, but sequencing and methylation profiling are not available, a final diagnosis of “Supratentorial ependymoma, NOS” would be appropriate. NEC, on the other hand, means that the appropriate molecular testing was performed but did not provide enough useful information for further classification. Thus, if molecular testing was performed on that supratentorial ependymoma but failed to uncover a ZFTA or YAP1 fusion, it would be called “Supratentorial ependymoma, NEC.”
Adult-Type Diffuse Gliomas
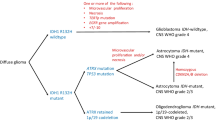
Diffuse gliomas, accounting for ~70% of adult brain tumors, are the most common type of primary brain tumor to arise in adults [6] (the most common CNS neoplasm overall is metastatic disease). Prior to 2016, morphologic features drove the classification of all diffuse gliomas. Tumors with round, uniform nuclei and cytoplasmic clearing were referred to as oligodendrogliomas, those with clumped chromatin and angulated nuclei were referred to as astrocytomas, and those with intermediate features were called oligoastrocytomas [7]. In the 2016 edition, molecular features were introduced into the classification of gliomas, with IDH mutation and 1p/19q codeletion required for the diagnosis of oligodendroglioma, and IDH mutation status and histologic grade used to parse astrocytomas into subtype [1]. In the 2021 classification, diffuse gliomas are now sorted into three basic types by morphology and molecular features with, grading done within each type. Hybrid entities like oligoastrocytoma, which are nearly always classified as other entities when molecular testing is performed, are no longer listed [8, 9].
One key difference between the 2016 and 2021 WHO classifications is in the way that IDH-wildtype gliomas are defined and graded. Although high-grade morphologic features such as mitoses, necrosis, and microvascular proliferation are still considered, all tumors lacking IDH mutations that have concomitant gain of chromosome 7 and loss of chromosome 10, EGFR amplification, or TERT promoter mutations are called glioblastoma and are given a WHO grade of 4 [10]. These tumors, which tend to occur in older adults and are rare below the age of 55, are highly aggressive, with death occurring within 15–18 months for most patients even with chemotherapy and radiation [11]. For most patients, symptoms related to mass effect develop rapidly, and high-grade imaging features, such as peripheral enhancement and central necrosis, are usually present at diagnosis. The current standard treatments include maximal surgical resection (when anatomically feasible), radiation, and temozolomide [12,13,14]. IDH wildtype glioblastoma is a morphologically, genetically heterogeneous category comprised of multiple different subtypes, including a small cell type (which mimics oligodendroglioma), a granular cell type with PAS-positive cytoplasmic inclusions, an epithelioid type with well-defined cytoplasmic borders and ample, eosinophilic cytoplasm, a giant cell type, and a sarcomatous type that may lose GFAP and olig2 expression and contain heterologous elements. By definition, IDH-wildtype glioblastomas are negative for IDH1 R132H, and the majority express markers of glial differentiation and retain normal ATRX by immunohistochemistry (meaning that no ATRX mutation is present). In addition to the aforementioned EGFR, TERT promoter, and + 7/-10 phenotypes in the diagnostic criteria, other common molecular abnormalities include CDKN2A/B deletion, PTEN alterations, TP53 mutations, MDM2 or MDM4 amplification, BRAF V600E mutations (especially the epithelioid subtype), and MGMT promoter methylation [15,16,17]. Of these, MGMT promoter methylation status is the most critical, as it predicts response to alkylating chemotherapeutic drugs such as temozolomide and lomustine [13, 18, 19]. MGMT promoter methylation status predicts both overall and progression-free survival in elderly patients with glioblastoma treated with alkylating agents in addition to radiotherapy [20]. PTEN status may also be important for predicting response to therapy. Some studies show that PTEN mutations, which are present in about 40% of gliomas, may render them more sensitive to radiation therapy [21]. One study suggested that, in patients with EGFR amplification, targeted EGFR inhibitors may only be effective in patients with intact PTEN expression [22]. A handful of case reports also suggest that BRAF inhibitors may be effective only when BRAF V600E mutations are present [23, 24]. More trials are needed in order to determine which groups of patients might benefit from targeted therapies.
Astrocytoma, IDH-mutant, is defined by a change-of-function mutation in IDH1 or IDH2 resulting in overproduction of the oncometabolite D-2-hydroxyglutarate, which acts as an inhibitor of enzymes that use α-ketoglutarate as a cofactor, such as certain DNA demethylases, resulting in genomic CpG hypermethylation and suppression of differentiation [25]. They most often occur in younger adults (median age 38) and are rarely diagnosed in adults over the age of 55. Patients usually present with seizures and are found to have diffuse, T2 FLAIR hyperintense, supratentorial masses with little or no enhancement [26]. The majority of IDH-mutant astrocytomas also have TP53 alterations resulting in strong nuclear accumulation of abnormal p53 in >50% of tumor cell nuclei. About 90% of supratentorial IDH-mutant astrocytomas also have ATRX mutations that result in loss of normal ATRX expression in the tumor cells [27, 28]. This makes p53 and ATRX immunostains useful as part of a panel when working up diffuse gliomas – a young patient with a glioma that displays strong p53 expression and loss of ATRX is likely to have a non-canonical IDH mutation if IDH1 R132H immunostain is negative [27]. IDH-mutant astrocytoma grades range from 2 to 4, based on the presence of anaplasia, mitotic activity, necrosis, microvascular proliferation, and homozygous CDKN2A/B deletion [29]. One major change between the 2016 and 2021 WHO Classifications is that, even when enough high-grade features are present to warrant a grade 4 designation, IDH-mutant astrocytomas are no longer referred to as glioblastomas, since even high-grade IDH-mutant astrocytomas are less aggressive than their IDH-wildtype counterparts [30]. Nevertheless, the current recommended standard of therapy for high-grade IDH-mutant gliomas remains similar to that for IDH-wildtype glioblastoma [12], although this may change as clinical trial target patients based on IDH status as well as tumor grade.
In addition to whole arm 1p/19q codeletion, IDH mutations are required for the diagnosis of oligodendroglioma, reflecting the fact that true 1p/19q codeletion occurring from unbalanced translocation is always seen in conjunction with IDH1 or IDH2 mutations [29, 31,32,33]. Like IDH-mutant astrocytomas, these are relatively less aggressive tumors that primarily occur in younger adults (median age 43), although gliomatosis cerebri-like growth patterns and seeding of the cerebrospinal fluid can sometimes be seen in more advanced stages. In addition to IDH mutations and 1p/19q codeletion, the majority of oligodendrogliomas also have TERT promoter mutations [32]. In contrast to IDH-mutant astrocytomas, oligodendrogliomas tend to have retained ATRX expression and lack accumulation of p53, since 1p/19q co-deletion is essentially mutually exclusive with TP53 and ATRX alterations [34, 35]. Some oligodendrogliomas have CDKN2A/B deletion, which predicts more aggressive behavior when present [36]. As was the case in the 2016 edition, grades range from 2 to 3 based on the presence of anaplasia, mitotic activity, necrosis, and microvascular proliferation [2]. Currently, radiation followed by adjuvant procarbazine, lomustine, and vincristine (PCV therapy) is the recommended treatment protocol for oligodendrogliomas [37, 38].
Pediatric-Type Diffuse Gliomas
Since the 2016 WHO Classification was published, understanding of the distinct biology of pediatric-type diffuse gliomas has exploded, resulting in the addition of distinct chapters containing eight newly added tumor types [2]. Four are classified as diffuse low-grade gliomas, reflecting their relatively indolent clinical behavior despite lack of a clear tumor: nontumor border. All are characterized by mutations that result in mitogen-activated protein kinase (MAPK) pathway activation and by the absence of IDH or histone mutations [39]. The first of these, “diffuse astrocytoma, MYB- or MYBL1-altered,” is defined by MYB- or MYBL1- fusions, with the most common fusion partners being PCDHGA1, MMP16, and MAML2 [40,41,42,43]. Angiocentric gliomas are only distinguishable from diffuse astrocytoma, MYB- or MYBL1-altered by clustering of tumor cells around blood vessels, and a different MYB fusion partner (usually QKI) [40,41,42, 44]. “Polymorphous low-grade neuroepithelial tumor of the young (PLNTY)” typically has a mixture of cells with oligodendroglial and astrocytic morphology with aberrant CD34 expression (Fig. 1), and frequently features perivascular rosettes and coarse calcifications. PLNTYs have a variety of MAPK-activating alterations, including BRAF V600E mutations, NTRK alterations, and fusions involving FGFR2 or FGFR3 [45]. “Diffuse low-grade glioma, MAPK pathway-altered” commonly has BRAF V600E mutations or FGFR1 alterations, much like both PLNTY and low-grade glioneuronal tumors (e.g., dysembryoplastic neuroepithelial tumor and ganglioglioma) [42, 46]. All four of these tumor types occur in the cerebral hemispheres, have low-grade features on imaging, often present with seizures, and show a predilection for teenagers or young adults [40, 42, 44]. All four also show closely related DNA methylation profiles [47], so it remains to be seen whether they will ultimately remain distinct tumor types in future editions of the WHO classification. All have targetable MAPK pathway alterations, and early studies using targeted therapies have been promising [48,49,50].
Polymorphous low-grade neuroepithelial tumor of the young. In keeping with the “polymorphous” descriptor, polymorphous low-grade neuroepithelial tumor of the young can have a variety of appearances, including that of a diffuse glioma (A), ependymoma (B), and oligodendroglioma (C). These tumors an have abundant mineralization (D) and show abundant CD34 positivity (E) and OLIG2 nuclear staining (F). This particular tumor had a BRAF V600E mutation, and clustered among PLNTYs by DNA methylation profiling
Four of the pediatric-type diffuse gliomas are classified as high-grade: “diffuse midline glioma, H3 K27-altered;” “diffuse hemispheric glioma, H3 G34-mutant;” “diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype;” “infant-type hemispheric glioma” [2, 26, 51]. The latter three were newly added to the 2021 edition of the WHO Classification. Like the low-grade pediatric gliomas, all four lack IDH mutations and should be considered in young adult patients with IDH wildtype gliomas. Both diffuse midline glioma, H3 K27-altered and diffuse hemispheric glioma, H3 G34-mutant are driven by histone mutations. For reasons that are not entirely understood, tumors with H3 K27 mutations tend to occur in midline structures, while those with H3 G34 mutations tend to occur in the cerebral hemispheres [52,53,54,55,56]. Although diffuse midline gliomas have somewhat variable morphology, with some having high proliferation rates and necrosis like the high-grade gliomas seen in adults (Fig. 2) and others lacking those features, they all have decreased H3K27Me3 by immunohistochemistry. In diffuse midline gliomas, such loss occurs by a lysine-to-methionine substitution in the histone H3 protein, resulting in inhibition of the EZH2 catalytic subunit of the polycomb repressive complex 2 (PRC2) protein [57]. In histone-mutated hemispheric gliomas, it occurs when substitution of a glycine at position 35 for arginine or valine results in reduced binding of SETD2 and KDM2A to the tail of the histone H3 protein, resulting in diminished H3 K37 Me3 [58, 59]. Both mechanisms lead to increased proliferation and decreased differentiation, and both histone-mutant gliomas are aggressive tumors with uniformly poor prognoses [51, 52, 60]. The third tumor, high-grade glioma, H3-wildtype and IDH-wildtype, is a category that encompasses high-grade diffuse gliomas that lack both histone and IDH mutations and can have multiple driver mutations (Fig. 3). Some have similar driver mutations as adult IDH-wildtype gliomas, including EGFR, PDGFRA, TP53, and NF1, but their methylation profiles are distinct from adult IDH-wildtype gliomas [61]. Three different subgroups have been identified by DNA methylation profiling: pHGG RTK1, pHGG RTK2, and pHGG MYCN. Tumors of the pHGG RTK1 subtype most frequently have PDGFRA alterations and are the type most frequently found in patients with Lynch syndrome. pHGG RTK2 tumors most frequently have EGFR amplification and TERT promoter mutations, while pHGG MYCN tumors have MYCN activation (usually amplification) [61]. Like pediatric high-grade gliomas with histone mutations, these tumors are highly aggressive with poor prognoses, with 2-year survival rate of 23.5% and median overall survival of only 17 months, even when MGMT promoter methylation is present [54]. Infant-type hemispheric glioma (Fig. 4) is a large, hemispheric mass that typically occurs during the first year of life and is usually driven by RTK-activating fusions, including those in the NTRK family, ROS1, ALK, or MET [62]. Despite their histopathologic similarity to IDH-wildtype glioblastomas, these have a better prognosis than the other three pediatric-type high-grade gliomas, with 5-year survival rates ranging from 25 to 50%, and there are some data suggesting that they respond to drugs targeting whichever RTK is altered [63].
Diffuse midline glioma, H3 K27-altered. Diffuse midline gliomas tend to look like most other diffusely infiltrative gliomas (A), including immunopositivity for GFAP (B) and OLIG2 (C). In keeping with their high-grade nature, Ki67 is usually quite high (D). H3 K27M-specific antibody often shows robust nuclear staining (E); those same tumor cells will be weak to negative for H3K27me3, whereas admixed nonneoplastic cells will still be positive (F). Scale bar = 50 microns in all panels
Diffuse pediatric-type high-grade glioma, H3-wildtype and IDH-wildtype. This diffuse pediatric-type high-grade glioma, H3 wildtype and IDH-wildtype, looked like a typical diffuse glioma infiltrating into surrounding brain tissue (A); many tumor cells will be Ki67 positive (B). This particular tumor had MYCN amplification and TP53 mutation, mapped to diffuse pediatric-type high-grade glioma, H3 wildtype, and IDH-wildtype by DNA methylation profiling, and had already disseminated throughout the cerebrospinal fluid at the time of clinical presentation. Scale bar = 50 microns in both panels
Infant-type hemispheric glioma. Morphologically, infant-type hemispheric gliomas may be indistinguishable from adult-type IDHwt glioblastomas, with palisading necrosis (A), abundant mitoses (B), variable GFAP positivity (C), and elevated Ki67 (D). However, they will not have the same molecular profile as glioblastomas; this tumor had an isolated NTRK fusion, and mapped to infant-type hemispheric glioma by DNA methylation profiling. Scale bar = 250 microns in A, 50 microns in B–D
Circumscribed Astrocytic Gliomas
Gliomas with more well-delineated borders separating them from the surrounding brain parenchyma, previously referred to as “other astrocytic tumors,” are now categorized as circumscribed astrocytic gliomas. This category includes pilocytic astrocytoma, subependymal giant cell tumor, pleomorphic xanthoastrocytoma, chordoid glioma (Table 1), “astroblastoma, MN1-altered,” and “high-grade astrocytoma with piloid features” [2, 51]. Most of the entities in this category are well established with only minor changes in the names, e.g., astroblastoma, MN1-altered and chordoid glioma. Astroblastomas, with their perivascular rosettes and reverse nuclear polarity, share some histomorphologic features with ependymal and embryonal tumors (Fig. 5) but are now defined by MN1 alterations [64,65,66]. Patients generally do well following surgical resection, but when anatomy precludes complete excision, chemotherapy and radiation offer some benefit [67]. Chordoid glioma (formerly known as “chordoid glioma of the third ventricle”), which nearly always occurs in the anterior third ventricle of adults, is comprised of spindled to epithelioid TTF1- and GFAP-positive cells in a myxoid stroma, a recurrent p.D463H missense mutation in the PRKCA gene, and is frequently separated from the adjacent brain parenchyma with a dense, lymphoplasmacytic infiltrate [68, 69].
Astroblastoma. Astroblastomas can show a variety of morphologies, including heavy sclerosis and mineralization (A). Tumor cells show variable GFAP immunopositivity (B) but are generally positive for OLIG2 (C), epithelial membrane antigen (D), podoplanin (E), and SATB2 (F). Scale bar in F = 100 microns in all panels
High-grade astrocytoma with piloid features, the only new circumscribed astrocytic glioma, is an aggressive astrocytic neoplasm with a combination of MAPK pathway-activating alterations (e.g., involving NF1, FGFR, or BRAF), ATRX mutations (manifesting as loss of normal ATRX immunostain), and homozygous CDKN2A/B deletion [70]. It can occur anywhere in the central nervous system but most often arises in the posterior fossa, and typically occurs in middle-aged adults [70]. The morphologic features are variable and can resemble glioblastoma or pleomorphic xanthoastrocytoma (PXA); features reminiscent of pilocytic astrocytoma, such as eosinophilic granular bodies, Rosenthal fibers, and long, delicate processes are only seen in a third of cases. While most of these tumors arise de novo, a small subset arise in pre-existing grade 1 pilocytic astrocytoma and were previously called “anaplastic pilocytic astrocytomas” [70]. Recent studies using genomic DNA methylation profiling revealed that high-grade astrocytoma with piloid features have their own distinct cluster [70]. There is only one retrospective study with any data on long-term prognosis, but the 5-year survival rate is roughly 50% and overall survival appears to be similar to that for patients with astrocytoma, IDH-mutant, WHO grade 4 [70]. As with some of the other more recently defined entities, it remains to be seen whether high-grade astrocytomas with piloid features respond better to conventional therapies versus targeted therapies.
Sometimes, it can be difficult to distinguish between circumscribed and diffuse astrocytic gliomas. PXAs, for example, can look very similar to epithelioid glioblastomas, especially if the PXA is grade 3 with numerous mitoses and/or necrosis. Both are IDH wildtype, both can have CDKN2A/B deletion and MAPK-activating alterations (e.g., BRAF V600E), and both can arise in similar locations and age groups. Genomic methylation profiling is particularly helpful in such circumstances, as many tumors thought to be epithelioid glioblastomas actually map either to PXA or pediatric high-grade glioma, RTK1 subtype [71].
Ependymal Tumors
Because of advanced molecular testing techniques such as DNA methylation profiling, categorization of ependymomas has completely changed from being based on morphology to being based on location and molecular alteration [72]. Tanycytic, clear cell, and papillary are listed as morphologic phenotypes rather than separate subtypes, and anaplastic ependymoma is no longer listed as an entity [2]. The majority of supratentorial ependymomas have fusions involving either ZFTA or YAP1 and are derived from radial glial cells [73]. ZFTA fusion-positive ependymomas are more common, comprising 25–58% of all supratentorial ependymomas in adults and 66–84% in children [74,75,76]. The most common fusion partner is RELA, and homozygous deletion of CKDN2A/B, which predicts more aggressive clinical behavior, may be present [77]. YAP1 fusion-positive ependymomas, which show more indolent behavior than ZFTA fusion-positive ependymomas, are rare and tend to occur primarily in young children [78]. Other genetic alterations predicting their clinical behavior have not yet been well studied. Either can be assigned a grade of 2 or 3, depending on the presence of mitotic activity and microvascular proliferation [79].
Posterior fossa ependymomas are sorted into PFA and PFB categories by DNA methylation profiling [80]. Like diffuse midline gliomas, PFA ependymomas show loss of H3 K27 trimethylation; however, only a small subset (~4%) have histone mutations [81]. Instead, PFA ependymomas express more Enhancer of Zest Homologs Inhibitory Protein (EZHIP), which binds to and inhibits the catalytic domain of PRC2 in a similar manner to the H3 K27M mutated histone protein [81, 82]. Such EZHIP overexpression also drives the rare diffuse midline gliomas that lack histone mutations, and in both PFA ependymomas and diffuse midline gliomas, H3 histone mutations and EZHIP alterations are mutually exclusive [83]. PFB ependymomas usually show whole chromosome abnormalities such as 22q loss, monosomy 6, and monosomy 18 [80, 84]. In addition, PFB ependymomas are more likely to occur in older adults and adolescents and are less aggressive than PFA ependymomas. Gain of chromosome 1q and incomplete resection are poor prognostic indicators for all types of posterior fossa ependymomas, and loss of 13q suggests worse behavior in PFB ependymomas [85].
Spinal ependymomas, which are their own cluster on DNA methylation profiling, have 22q loss like PFB ependymomas [84]. These occur more frequently as intramedullary masses in the cervicothoracic region of the spinal cord, in contrast to myxopapillary ependymoma, which occur in the lumbar region [86]. For the most part, spinal ependymomas have good outcomes and long progression free survival times, except for the subset with MYCN amplification, which spread throughout the CSF [87]. Myxopapillary ependymoma has its own distinct methylation cluster and was historically given a WHO grade of 1 due to its generally good prognosis and bland appearance [1]. But, because of the possibility of CSF dissemination, which is more common in younger patients, and because so many patients live with persistent disease requiring multiple operations and radiotherapy, they are now considered WHO grade 2 in the 2021 classification [88,89,90].
Subependymomas, which are still considered WHO grade 1, are indolently growing glial tumors with subependymal morphology. Approximately two-thirds of them arise in the fourth ventricle, with the remainder being supratentorial [91]. Like ependymomas, they can show cystic changes and calcification on imaging but are less likely to show much enhancement after contrast administration [92]. The most common genetic alterations are the loss of chromosome 19, partial loss of chromosome 6, and even H3 K27 mutations, all three of which can occur in brainstem subependymomas [84]. Overall, the clinical behavior of these tumors is benign with occurrence being rare even after subtotal resection, even when H3 K27 mutations are present [91, 92].
Embryonal Tumors
Like pediatric gliomas and ependymal tumors, DNA methylation profiling has resulted in an explosion in the number of embryonal tumor types, both from the splitting of existing tumor types into additional categories and from the addition of newly characterized types of tumors [93, 94]. Medulloblastomas, the most common embryonal tumors, are divided into four different molecular subtypes, including WNT-activated, SHH-activated and TP53-wildtype, SHH-activated and TP53-mutant, and non-WNT/non-SHH. Pediatric WNT-activated medulloblastomas respond extremely well to existing therapies and have excellent prognoses, but retain a WHO grade of 4 to reflect their aggressive nature without treatment [93, 95,96,97]. SHH-activated tumors are more aggressive if TP53 mutations or MYCN amplification are present [96]. Non-WNT/non-SHH tumors, formerly known as group 3 and group 4 medulloblastomas, comprise the majority of medulloblastomas. As is the case with SHH-activated tumors, MYCN amplification is a poor prognostic indicator [98]. Non-WNT/Non-SHH tumors can be further divided into 8 different categories by DNA methylation profiling, with subgroups 2, 3, and 8 having the worst outcomes and groups 6 and 7, which are more likely to have favorable cytogenetic aberrations, such as gain of chromosome 7 and loss of chromosomes 8 or 11, having the best [93].
Three new entities have been added to embryonal tumors. The first of these, cribriform neuroepithelial tumor, is a provisional entry. It tends to occur in periventricular locations and has SMARCB1 mutations and INI1 loss, much like atypical teratoid rhabdoid tumors [99, 100]. Unlike atypical teratoid rhabdoid tumor, however, cribriform neuroepithelial tumors lack rhabdoid morphology and have favorable response to therapy with average survival times of approximately 10 years [99]. CNS neuroblastoma, FOXR2-activated, consists of sheets of small, mitotically active, primitive cells with high N/C ratios that form occasional rosettes (Fig. 6). Like neuroblastomas, they can harbor areas of neuropil and ganglion cells. They are rare and newly classified, so there is currently little data on their prognosis [101]. CNS tumor with BCOR internal tandem duplication is defined by a heterozygous internal tandem duplication in exon 15 of BCOR [102]. They have small, uniform, ovoid to spindle-shaped cells, often arranged in perivascular pseudorosettes that can easily be confused with those in ependymal tumors or astroblastomas (Fig. 7), and can also have palisading necrosis reminiscent of that in glioblastomas. BCOR internal tandem duplications do not co-occur with ZFTA or YAP1 fusions, MN1 alterations, IDH mutations, or H3 mutations, and DNA methylation profiling can be used to reliably distinguish them from other morphologically similar entities [102]. These tumors are rare, which limits prognostic data, but case series suggest the overall prognosis to be poor [102].
CNS neuroblastoma, FOXR2-activated. CNS neuroblastoma with FOXR2-activation features sheets of tumor cells with small round nuclei and a high nuclear:cytoplasmic ratio like most other embryonal tumors, although the tumor shown here had more abundant neuropil (A). Like many other new tumor entities, these tumor cells often arrange themselves in a perivascular pattern (B). Most such tumors will also show other high-grade features like necrosis and mitoses, but this one did not. Still, it clearly mapped to CNS neuroblastoma with FOXR2-activation by DNA methylation profiling. Scale bar = 250 microns in A, 100 microns in B
Meningioma
Meningothelial tumors are all categorized as one heterogeneous type, with 15 distinct morphologic subtypes that range in WHO grade from 1 to 3 [2]. Numerous studies characterizing the molecular alterations seen in meningiomas, and how they correspond to location and morphology, have been performed [103,104,105,106]; however, meningiomas are still mostly graded the same way they were in the 2016 classification with two exceptions. Since TERT promoter mutations and homozygous deletion of CDKN2A/B have been associated with more aggressive clinical behavior, these are now sufficient for a WHO grade of 3 even if the histopathology does not otherwise meet the criteria [107, 108]. Complete resection is still the primary way meningiomas are managed; however, mutations known to lead to more aggressive behavior can be used to help guide the decision for adjuvant radiation or chemotherapy.
Conclusions
The 2021 WHO CNS tumor classification draws on a wealth of data from molecular testing that led to the creation of new entities and more accurate stratification of pre-existing entities. Future clinical trials can recruit more homogeneous categories of patients, which will allow for better understanding of how tumor biology corresponds to treatment response and for the development of more targeted therapies. However, more multi-institutional and multi-national studies will be needed to address the less common tumor types. More long-term prognostic studies will also be needed to better characterize some of the newer entities.
References
Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51.
Horbinski C, Ligon KL, Brastianos P, Huse JT, Venere M, Chang S, et al. The medical necessity of advanced molecular testing in the diagnosis and treatment of brain tumor patients. Neuro Oncol. 2019;21(12):1498–508.
Louis DN, Aldape K, Brat DJ, Capper D, Ellison DW, Hawkins C, et al. Announcing cIMPACT-NOW: the Consortium to inform molecular and practical approaches to CNS tumor taxonomy. Acta Neuropathol. 2017;133(1):1–3.
Louis DN, Wesseling P, Paulus W, Giannini C, Batchelor TT, Cairncross JG, et al. cIMPACT-NOW update 1: not otherwise specified (NOS) and not elsewhere classified (NEC). Acta Neuropathol. 2018;135(3):481–4.
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(Supplement_1):iv1–96.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.
Hinrichs BH, Newman S, Appin CL, Dunn W, Cooper L, Pauly R, et al. Farewell to GBM-O: genomic and transcriptomic profiling of glioblastoma with oligodendroglioma component reveals distinct molecular subgroups. Acta Neuropathol Commun. 2016;4(1).
Sahm F, Reuss D, Koelsche C, Capper D, Schittenhelm J, Heim S, et al. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014;128(4):551–9.
Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” Acta Neuropathol. 2018;136(5):805–10.
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–100.
Van Den Bent MJ, Tesileanu CMS, Wick W, Sanson M, Brandes AA, Clement PM, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053–22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(6):813–23.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Nabors LB, Portnow J, Ahluwalia M, Baehring J, Brem H, Brem S, et al. Central nervous system cancers, Version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18(11):1537–70.
Cancer Genome Atlas (TCGA) Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–77.
Kleinschmidt-Demasters BK, Aisner DL, Foreman NK. BRAF VE1 Immunoreactivity patterns in epithelioid glioblastomas positive for BRAF V600E mutation. Am J Surg Pathol. 2015;39(4):528–40.
Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La Cruz C, Sulman EP, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12(2):116–21.
Binabaj MM, Bahrami A, ShahidSales S, Joodi M, Joudi Mashhad M, Hassanian SM, et al. The prognostic value of MGMT promoter methylation in glioblastoma: a meta-analysis of clinical trials. J Cell Physiol. 2018;233(1):378–86.
Reifenberger G, Hentschel B, Felsberg J, Schackert G, Simon M, Schnell O, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131(6):1342–50.
Ma J, Benitez JA, Li J, Miki S, De Albuquerque CP, Galatro T, et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell. 2019;35(3):504-18.e7.
Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–24.
Kanemaru Y, Natsumeda M, Okada M, Saito R, Kobayashi D, Eda T, et al. Dramatic response of BRAF V600E-mutant epithelioid glioblastoma to combination therapy with BRAF and MEK inhibitor: establishment and xenograft of a cell line to predict clinical efficacy. Acta Neuropathol Commun. 2019;7(1).
Woo PYM, Lam TC, Pu JKS, Li LF, Leung RCY, Ho JMK, et al. Regression of BRAF (V600E) mutant adult glioblastoma after primary combined BRAF-MEK inhibitor targeted therapy: a report of two cases. Oncotarget. 2019;10(38):3818–26.
Unruh D, Zewde M, Buss A, Drumm MR, Tran AN, Scholtens DM, et al. Methylation and transcription patterns are distinct in IDH mutant gliomas compared to other IDH mutant cancers. Sci Rep. 2019;9(1).
Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma. IDH-mutant Acta Neuropathologica. 2018;135(4):639–42.
Ebrahimi A, Skardelly M, Bonzheim I, Ott I, Mühleisen H, Eckert F, et al. ATRX immunostaining predicts IDH and H3F3A status in gliomas. Acta Neuropathol Commun. 2016;4(1).
Takami H, Yoshida A, Fukushima S, Arita H, Matsushita Y, Nakamura T, et al. Revisiting TP53 mutations and immunohistochemistry–a comparative study in 157 diffuse gliomas. Brain Pathol. 2015;25(3):256–65.
Brat DJ, Aldape K, Colman H, Figrarella-Branger D, Fuller GN, Giannini C, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603–8.
Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136(1):153–66.
Labussiere M, Idbaih A, Wang XW, Marie Y, Boisselier B, Falet C, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74(23):1886–90.
Yip S, Butterfield YS, Morozova O, Chittaranjan S, Blough MD, An J, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226(1):7–16.
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–508.
Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–68.
Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–98.
Appay R, Dehais C, Maurage C-A, Alentorn A, Carpentier C, Colin C, et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro-Oncology. 2019.
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–43.
Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus Procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–55.
Ellison DW, Hawkins C, Jones DTW, Onar-Thomas A, Pfister SM, Reifenberger G, et al. cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol. 2019;137(4):683–7.
Johnson A, Severson E, Gay L, Vergilio J-A, Elvin J, Suh J, et al. Comprehensive genomic profiling of 282 pediatric low- and high-grade gliomas reveals genomic drivers, tumor mutational burden, and hypermutation signatures. Oncologist. 2017;22(12):1478–90.
Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci. 2013;110(20):8188–93.
Ryall S, Zapotocky M, Fukuoka K, Nobre L, Guerreiro Stucklin A, Bennett J, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569-83.e5.
Wefers AK, Stichel D, Schrimpf D, Coras R, Pages M, Tauziède-Espariat A, et al. Isomorphic diffuse glioma is a morphologically and molecularly distinct tumour entity with recurrent gene fusions of MYBL1 or MYB and a benign disease course. Acta Neuropathol. 2020;139(1):193–209.
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–12.
Huse JT, Snuderl M, Jones DTW, Brathwaite CD, Altman N, Lavi E, et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133(3):417–29.
Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131(6):833–45.
Pratt D, Sahm F, Aldape K. DNA methylation profiling as a model for discovery and precision diagnostics in neuro-oncology. Neuro Oncol. 2021;23(Supplement_5):S16–29.
Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF Inhibition in BRAFV600-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36(35):3477–84.
Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–22.
Sait SF, Gilheeney S, Bale T, Rosenblum M, Haque S, Ibanez K, et al. EPCT-10. DEBIO1347, an oral fgfr inhibitor: results from a single center study in recurrent/refractory fgfr altered pediatric gliomas. Neuro Oncol. 2021;23(1):i48–9.
Louis DN, Wesseling P, Aldape K, Brat DJ, Capper D, Cree IA, et al. cIMPACT-NOW update 6: new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020;30(4):844–56.
Roux A, Pallud J, Saffroy R, Edjlali-Goujon M, Debily M-A, Boddaert N, et al. High-grade gliomas in adolescents and young adults highlight histomolecular differences from their adult and pediatric counterparts. Neuro Oncol. 2020;22(8):1190–202.
Chan K-M, Fang D, Gan H, Hashizume R, Yu C, Schroeder M, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–90.
Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520-37.e5.
Korshunov A, Capper D, Reuss D, Schrimpf D, Ryzhova M, Hovestadt V, et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol. 2016;131(1):137–46.
Chen CCL, Deshmukh S, Jessa S, Hadjadj D, Lisi V, Andrade AF, et al. Histone H3.3G34-mutant interneuron progenitors Co-opt PDGFRA for gliomagenesis. Cell. 2020;183(6):1617-33.e22.
Lewis PW, Müller MM, Koletsky MS, Cordero F, Lin S, Banaszynski LA, et al. Inhibition of PRC2 Activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–61.
Yang S, Zheng X, Lu C, Li G-M, Allis CD, Li H. Molecular basis for oncohistone H3 recognition by SETD2 methyltransferase. Genes Dev. 2016;30(14):1611–6.
Cheng Z, Cheung P, Kuo AJ, Yukl ET, Wilmot CM, Gozani O, et al. A molecular threading mechanism underlies Jumonji lysine demethylase KDM2A regulation of methylated H3K36. Genes Dev. 2014;28(16):1758–71.
Guidi M, Giunti L, Buccoliero A, Caporalini C, Censullo M, Galli L, et al. Genetic signature and treatment of pediatric high‑grade glioma. Mol Clin Oncol. 2021;14(4).
Korshunov A, Schrimpf D, Ryzhova M, Sturm D, Chavez L, Hovestadt V, et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017;134(3):507–16.
Guerreiro Stucklin AS, Ryall S, Fukuoka K, Zapotocky M, Lassaletta A, Li C, et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun. 2019;10(1).
Clarke M, Mackay A, Ismer B, Pickles JC, Tatevossian RG, Newman S, et al. Infant high-grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Cancer Discov. 2020;10(7):942–63.
Chen W, Soon YY, Pratiseyo PD, Sutanto R, Hendriansyah L, Kuick CH, et al. Central nervous system neuroepithelial tumors with MN1-alteration: an individual patient data meta-analysis of 73 cases. Brain Tumor Pathol. 2020;37(4):145–53.
Lehman NL, Usubalieva A, Lin T, Allen SJ, Tran QT, Mobley BC, et al. Genomic analysis demonstrates that histologically-defined astroblastomas are molecularly heterogeneous and that tumors with MN1 rearrangement exhibit the most favorable prognosis. Acta Neuropathol Commun. 2019;7(1).
Wood MD, Tihan T, Perry A, Chacko G, Turner C, Pu C, et al. Multimodal molecular analysis of astroblastoma enables reclassification of most cases into more specific molecular entities. Brain Pathol. 2018;28(2):192–202.
Burford A, Mackay A, Popov S, Vinci M, Carvalho D, Clarke M, et al. The ten-year evolutionary trajectory of a highly recurrent paediatric high grade neuroepithelial tumour with MN1:BEND2 fusion. Sci Rep. 2018;8(1).
Rosenberg S, Simeonova I, Bielle F, Verreault M, Bance B, Le Roux I, et al. A recurrent point mutation in PRKCA is a hallmark of chordoid gliomas. Nat Commun. 2018;9(1).
Horbinski C, Dacic S, McLendon RE, Cieply K, Datto M, Brat DJ, et al. Chordoid glioma: a case report and molecular characterization of five cases. Brain Pathol. 2009;19(3):439–48.
Reinhardt A, Stichel D, Schrimpf D, Sahm F, Korshunov A, Reuss DE, et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136(2):273–91.
Korshunov A, Chavez L, Sharma T, Ryzhova M, Schrimpf D, Stichel D, et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018;28(5):656–62.
Ellison DW, Aldape KD, Capper D, Fouladi M, Gilbert MR, Gilbertson RJ, Hawkins C, Merchant TE, Pajtler K, Venneti S, Louis DN. cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol. 2020;30(5):863–6.
Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–35.
Arabzade A, Zhao Y, Varadharajan S, Chen H-C, Jessa S, Rivas B, et al. ZFTA–RELA dictates oncogenic transcriptional programs to drive aggressive supratentorial ependymoma. Cancer Discov. 2021;11(9):2200–15.
Pagès M, Pajtler KW, Puget S, Castel D, Boddaert N, Tauziède-Espariat A, et al. Diagnostics of pediatric supratentorial RELA ependymomas: integration of information from histopathology, genetics, DNA methylation and imaging. Brain Pathol. 2019;29(3):325–35.
Pietsch T, Wohlers I, Goschzik T, Dreschmann V, Denkhaus D, Dörner E, et al. Supratentorial ependymomas of childhood carry C11orf95–RELA fusions leading to pathological activation of the NF-κB signaling pathway. Acta Neuropathol. 2014;127(4):609–11.
Jünger ST, Andreiuolo F, Mynarek M, Wohlers I, Rahmann S, Klein-Hitpass L, et al. CDKN2A deletion in supratentorial ependymoma with RELA alteration indicates a dismal prognosis: a retrospective analysis of the HIT ependymoma trial cohort. Acta Neuropathol. 2020;140(3):405–7.
Neumann JE, Spohn M, Obrecht D, Mynarek M, Thomas C, Hasselblatt M, et al. Molecular characterization of histopathological ependymoma variants. Acta Neuropathol. 2020;139(2):305–18.
Ellison DW, Kocak M, Figarella-Branger D, Felice G, Catherine G, Pietsch T, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10(1):7.
Witt H, Mack SC, Ryzhova M, Bender S, Sill M, Isserlin R, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell. 2011;20(2):143–57.
Pajtler KW, Wen J, Sill M, Lin T, Orisme W, Tang B, et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol. 2018;136(2):211–26.
Jain SU, Do TJ, Lund PJ, Rashoff AQ, Diehl KL, Cieslik M, et al. PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun. 2019;10(1).
Castel D, Kergrohen T, Tauziède-Espariat A, Mackay A, Ghermaoui S, Lechapt E, et al. Histone H3 wild-type DIPG/DMG overexpressing EZHIP extend the spectrum diffuse midline gliomas with PRC2 inhibition beyond H3–K27M mutation. Acta Neuropathol. 2020;139(6):1109–13.
Patjler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F, et al. Molecular classification of ependymal tumors across All CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–43.
Godfraind C, Kaczmarska JM, Kocak M, Dalton J, Wright KD, Sanford RA, et al. Distinct disease-risk groups in pediatric supratentorial and posterior fossa ependymomas. Acta Neuropathol. 2012;124(2):247–57.
Koeller KK, Rosenblum RS, Morrison AL. Neoplasms of the spinal cord and filum terminale: radiologic-pathologic correlation. Radiographics. 2000;20(6):1721–49.
Swanson AA, Raghunathan A, Jenkins RB, Messing-Jünger M, Pietsch T, Clarke MJ, et al. Spinal cord ependymomas with MYCN amplification show aggressive clinical behavior. J Neuropathol Exp Neurol. 2019;78(9):791–7.
Weber DC, Wang Y, Miller R, Villa S, Zaucha R, Pica A, et al. Long-term outcome of patients with spinal myxopapillary ependymoma: treatment results from the MD Anderson Cancer Center and institutions from the Rare Cancer Network. Neuro Oncol. 2015;17(4):588–95.
Bates JE, Choi G, Milano MT. Myxopapillary ependymoma: a SEER analysis of epidemiology and outcomes. J Neurooncol. 2016;129(2):251–8.
Bandopadhayay P, Silvera VM, Ciarlini PDSC, Malkin H, Bi WL, Bergthold G, et al. Myxopapillary ependymomas in children: imaging, treatment and outcomes. J Neurooncol. 2016;126(1):165–74.
Rushing EJ, Cooper PB, Quezado M, Begnami M, Crespo A, Smirniotopoulos JG, et al. Subependymoma revisited: clinicopathological evaluation of 83 cases. J Neurooncol. 2007;85(3):297–305.
Bi Z, Ren X, Zhang J, Jia W. Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J Neurosurg. 2015;122(1):49–60.
Sharma T, Schwalbe EC, Williamson D, Sill M, Hovestadt V, Mynarek M, et al. Second-generation molecular subgrouping of medulloblastoma: an international meta-analysis of Group 3 and Group 4 subtypes. Acta Neuropathol. 2019;138(2):309–26.
Kumar R, Liu APY, Orr BA, Northcott PA, Robinson GW. Advances in the classification of pediatric brain tumors through DNA methylation profiling: from research tool to frontline diagnostic. Cancer. 2018;124(21):4168–80.
Waszak SM, Northcott PA, Buchhalter I, Robinson GW, Sutter C, Groebner S, et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: a retrospective genetic study and prospective validation in a clinical trial cohort. Lancet Oncol. 2018;19(6):785–98.
Zhukova N, Ramaswamy V, Remke M, Pfaff E, Shih DJH, Martin DC, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–35.
Northcott PA, Shih DJH, Peacock J, Garzia L, Sorana Morrissy A, Zichner T, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56.
Schwalbe EC, Lindsey JC, Nakjang S, Crosier S, Smith AJ, Hicks D, et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. Lancet Oncol. 2017;18(7):958–71.
Johann PD, Hovestadt V, Thomas C, Jeibmann A, Heß K, Bens S, et al. Cribriform neuroepithelial tumor: molecular characterization of a SMARCB1-deficient non-rhabdoid tumor with favorable long-term outcome. Brain Pathol. 2017;27(4):411–8.
Gessi M, Japp AS, Dreschmann V, Zur Mühlen A, Goschzik T, Dörner E, et al. High-resolution genomic analysis of cribriform neuroepithelial tumors of the central nervous system. J Neuropathol Exp Neurol. 2015;74(10):970–4.
Sturm D, Orr B, Toprak U, Hovestadt V, Jones DT, Capper D, et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell. 2016;164(5):1060–72.
Ferris SP, Velazquez Vega J, Aboian M, Lee JC, Van Ziffle J, Onodera C, et al. High-grade neuroepithelial tumor with BCOR exon 15 internal tandem duplication—a comprehensive clinical, radiographic, pathologic, and genomic analysis. Brain Pathol. 2020;30(1):46–62.
Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597(7874):119–25.
Nassiri F, Mamatjan Y, Suppiah S, Badhiwala JH, Mansouri S, Karimi S, et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol. 2019;21(7):901–10.
Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–94.
Maas SLN, Stichel D, Hielscher T, Sievers P, Berghoff AS, Schrimpf D, et al. Integrated molecular-morphologic meningioma classification: a multicenter retrospective analysis, retrospectively and prospectively validated. J Clin Oncol. 2021;39(34):3839–52.
Boström J, Meyer-Puttlitz B, Wolter M, Blaschke B, Weber RG, Lichter P, et al. Alterations of the tumor suppressor genes CDKN2A (p16), p14, CDKN2B (p15), and CDKN2C (p18) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159(2):661–9.
Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–9.
Funding
CH was supported by National Institute for Health R01NS102669, R01NS117104, R01NS118039, the Northwestern University P50CA221747 SPORE in Brain Tumor Research, and the Lou and Jean Malnati Brain Tumor Institute at Northwestern.
Author information
Authors and Affiliations
Contributions
Disclosure forms provided by the authors are available with the online version of this article.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Invited review, “Special Issue on Therapeutic Advances in Neuro-oncology”.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smith, H.L., Wadhwani, N. & Horbinski, C. Major Features of the 2021 WHO Classification of CNS Tumors. Neurotherapeutics 19, 1691–1704 (2022). https://doi.org/10.1007/s13311-022-01249-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01249-0