Abstract
Human neuroimaging has had a major impact on the biological understanding of epilepsy and the relationship between pathophysiology, seizure management, and outcomes. This review highlights notable recent advancements in hardware, sequences, methods, analyses, and applications of human neuroimaging techniques utilized to assess epilepsy. These structural, functional, and metabolic assessments include magnetic resonance imaging (MRI), positron emission tomography (PET), and magnetoencephalography (MEG). Advancements that highlight non-invasive neuroimaging techniques used to study the whole brain are emphasized due to the advantages these provide in clinical and research applications. Thus, topics range across presurgical evaluations, understanding of epilepsy as a network disorder, and the interactions between epilepsy and comorbidities. New techniques and approaches are discussed which are expected to emerge into the mainstream within the next decade and impact our understanding of epilepsies. Further, an increasing breadth of investigations includes the interplay between epilepsy, mental health comorbidities, and aberrant brain networks. In the final section of this review, we focus on neuroimaging studies that assess bidirectional relationships between mental health comorbidities and epilepsy as a model for better understanding of the commonalities between both conditions.
Similar content being viewed by others
Introduction
Noninvasive neuroimaging tools have had a major impact on the understanding of the biological basis of human epilepsy and the relationship between pathology and the outcomes of interventions. Among the commonly utilized techniques are computerized tomography (CT), magnetic resonance imaging (MRI), electroencephalography (EEG), positron emission tomography (PET), spectroscopy (SPECT), and magnetoencephalography (MEG). Further advancements in human neuroimaging methods have afforded promising new insights into the biological basis of epilepsy and, importantly, our understanding of epilepsy as a network disorder. New techniques and approaches expected to emerge into the mainstream within the next decade will greatly influence our understanding of the biological basis of epilepsies and the mechanisms involved in symptom changes. In particular, investigations increasingly focus on epilepsy as a network disorder and in the context of comorbidities. For example, recent work has examined mental health comorbidities in epilepsies that are also related to aberrant brain function.
Network neuroimaging approaches have shown that focal epilepsies are characterized by distal and distributed disruptions in connectivity, function, and neurobiology [1, 2]. A similar recent shift in emphasis on brain network models is noted among psychopathologies [3, 4], including mood and anxiety disorders [5, 6] which share a high rate of comorbidity with epilepsies [7]. Together, novel neuroimaging approaches hold great potential for elucidating the bidirectional biological basis of mental health dysfunction and epilepsy [8]. In this review, noteworthy studies were included that we believe highlight promising new directions and important recent applications of novel imaging techniques on an editorial basis. In particular, we explore recent advances in whole-brain human neuroimaging approaches to understanding epilepsy as a distributed network disorder. This will encompass both new techniques to assess distributed network alterations, as well as tools that can be utilized to better assess the extent and intensity of neuroanatomical, functional, and physiological properties within discrete nodes or regions of these networks. In the final section of this review, we focus on the effects of epilepsy on the networks associated with mood and anxiety disorders and vice versa as a way of linking both states.
Magnetic Resonance Imaging
Due to an ideal balance of relative abundance, spatial resolution, and non-invasive ability to assess a variety of tissue or biochemical properties, MRI has emerged as a broadly utilized tool in the study of epilepsy [9]. From a network neuroimaging perspective, MRI affords a relatively direct whole-brain approach that is not possible with non-invasive EEG, near-infrared spectroscopy (NIRS), or MEG [10]. Among the most common MR techniques (Table 1) to study changes in whole-brain networks linked to epilepsy phenotypes are assessments of anatomical structure (volumetric and morphometric MRI), white matter tissue properties (diffusion MRI), neuronal activation (functional MRI), and metabolite concentrations (MR spectroscopic imaging (MRSI)). However, more recent developments in magnetic field strength (high field 7 T MR imaging), acquisition and sequence optimization (multi-band echo planar imaging (EPI), MRI fingerprinting), physical mathematical modeling (neurite compartment modeling, MRSI thermometry), and sophisticated statistical analysis algorithms (graph theory, machine learning) have expanded the utility of MRI in assessing changes in human brain networks.
Volumetric and Morphometric Magnetic Resonance Imaging
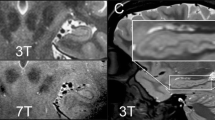
T1-weighted MR imaging is known for its relatively increased sensitivity to assess soft-tissue properties in epileptogenic lesions [11, 12]. Hippocampal volume loss, signal changes, and loss of internal architecture are hallmarks of medial temporal lobe sclerosis associated with temporal lobe epilepsy (TLE) [13,14,15]. However, from a research perspective, evaluation of subtler abnormalities among volumetric structure offers additional insights into the networks affected in TLE. Traditional volumetric studies assess relative changes in regional volume using atlas-based segmentation methods. However, continued development of morphometric analysis techniques builds on these traditional methods by assessing changes in the arrangement of nuclei and subnuclei within a region to provide fine cortical pattern information not necessarily related to increases in volume [16]. Thus, although morphometry is an important part of volumetric studies, some morphometry studies extend beyond assessing regional volumetric changes to visualize and quantify changes in specific shape patterns and internal architecture of brain structures (e.g., dentation of the hippocampus). Visualization and quantification of fine cortical anomalies and changes in structural patterns of hippocampal nuclei and subnuclei has been made possible through optimization of T1- or T2- weighted imaging at 3 T (3 T) and 7 T MRI and/or introduction of new data collection schemes resulting in improved spatial resolution of the collected images [17]. Specifically, morphometric analysis of hippocampal dentations of the inferior surface has shown that greater dentation relates to better episodic memory performance in healthy controls [16]. Similar methods have recently been utilized to quantify smooth or mildly irregular hippocampi in TLE, suggesting that asymmetry in dentation may serve as a hallmark of TLE phenotypes [18]. Additionally, subsequent reconstruction of anatomical information acquired during T2-weighted imaging is susceptible to artificial smoothing during initial processing of the MRI data at 3 T [17]. However, the increased spatial resolution of 7 T imaging combined with automated segmentation methods can more accurately and precisely estimate internal architecture of the hippocampi [19].Thus, new understanding about the role of hippocampal subfields and dentation patterns in patient profiles and as predictors of surgical outcomes may be possible [20]. Further, 7 T MRI has demonstrated an increased detection of focal cortical dysplasia (FCD) that can improve surgical outcomes in patients who were previously deemed MRI-negative [21, 22]. New developments in automated detection of epileptogenic lesions may lead to improved surgical outcomes. For example, morphometric analysis program (MAP) is a practical and valuable tool to guide the search for subtle cortical abnormalities among T1-weighted imaging for otherwise MRI-negative patients [23, 24]. Substantial additional benefits of the MAP tool include robustness to lower field strengths (i.e., 1.5 T MRI), retrospective assessment on presurgical MRI data to compare resection overlap and long-term outcomes, and an automated objective assessment of lesion extents [25]. Clinically, MAP has been utilized for detection of focal cortical dysplasia [26] and in prospective presurgical evaluations of epileptogenic lesions with encouraging preliminary results [27]. Likewise, when combined with machine learning algorithms, T1-weighted structural imaging has broadened detection of subtle morphometric changes among MRI-negative patients [28, 29]. Future developments are anticipated in the utilization of 7 T MRI and the vast increases in spatial resolution that accompany high field MRI in humans. For example, a recent retrospective study on MAP lesion detection suggests a major benefit of high-field MRI in detecting subtle FCD lesions of otherwise MRI-negative treatment-resistant epilepsy patients [22]. Additionally, high spatial resolution in 7 T MRI may be used to non-invasively discriminate between myelin loss in temporal white matter and cortical dysplasia in TLE, previously only detectable in resected histopathological samples [30]. Limiting factors in the high field human MRI domain are increased specific absorption rate (SAR), relaxation times, susceptibility artifacts, and physiological sensory side effects (e.g., vestibular, gustatory) as well as lower clinical utilization, bore circumference, radiofrequency field homogeneity, and availability of 7 T MRIs among research institutions [31]. However, these limiting factors may be partially mitigated by further demonstration of benefits, increases in bore circumference, and broader availability.
Magnetic Resonance Fingerprinting
MR fingerprinting (MRF) is a new approach to MR data acquisition sequences and processing that could enable more comprehensive tissue diagnosis and development of imaging biomarkers in epilepsies. Specifically, MRF utilizes a single and time-efficient multicontrast acquisition sequence that measures unique combinations of signal responses (i.e., fingerprints) as a function of multiple tissue properties [32]. MRF holds great potential as a more sophisticated assessment of epileptogenic lesions, such as malformations that occur at the interface between gray and white matter tissues [33]. Initial findings have shown that MRF improves accuracy in detection of lesions within gray and white matter portions of medial temporal areas [34, 35]. However, future studies are needed to determine whether this enhanced detection will benefit pre-surgical evaluation and therapeutic interventions.
Diffusion Magnetic Resonance Imaging
Diffusion-weighted MRI (DWI) relies on the Brownian motion of water molecules and relative permeability of lipids and other neuronal components to index the regional brain tissue properties, particularly white matter microstructure and structural connectivity. The traditional approach has been to model movement of water molecules within and between cell membranes based on assumptions of a Gaussian-based tensor-fitting model (diffusion tensor imaging (DTI)). As a post-processing method, DTI takes advantage of diffusion properties of the tissue to show unrestricted (isotropic) or restricted (anisotropic) water movement that can be modeled with a tensor resulting in eigenvectors (ε) and eigenvalues (λ) at every voxel. Next, eigenvalue measures based on the anisotropy of water movement yield a principle axis (λ1) and two secondary axes (λ2, λ3). These lambdas are utilized to then calculate axial diffusivity as an index of axonal integrity, radial diffusivity as an index of demyelination, and fractional anisotropy or mean diffusivity as an index of white matter health or integrity [36,37,38]. However, DTI is unable to discriminate between regions with isotropic movement due to an abundance of water with little to no cell membranes versus a large degree of axonal dispersion related to two or more crossing fibers [39]. Despite this limitation, DTI continues to provide new and valuable contributions to the understanding of epilepsy. In particular, a recent ENIGMA-Epilepsy (a large consortium dedicated to aggregating neuroimaging data) study utilized thousands of patient DTI scans to rank the most robust white matter microstructural differences, demonstrating syndrome-specific fractional anisotropy and mean diffusivity that may explain cognitive and psychiatric comorbidities or be used to guide treatment decisions [40]. Thus, while DTI has provided us with insights into the white matter integrity, traditional measures of diffusivity and anisotropy are limited by lack of biological specificity [41,42,43]. Another recent DTI study indicated that pre-operative tissue characteristics including fiber quantification could aid and provide better precision to predicting surgical outcomes of patients with TLE [44].
The abovementioned limitations in DTI have been mitigated by recently developed post-processing methods utilizing diffusion sequences with richer data acquisition, known as high angular resolution sequences (HARDI) multishell sequences. To address the limitation of crossing fibers and provide more accurate reconstructions of white mater fiber pathways throughout the brain, diffusion spectrum imaging (DSI) approach implements a multi-directional model instead of the bi-directional tensor model [45]. By combining DSI reconstruction with quantification of reconstructed white matter fibers, known as streamlines, increased neurodegenerative changes have been demonstrated recently in TLE patients [46]. Similar to DSI modeling that refines upon prior DTI-based approaches, diffusion kurtosis imaging (DKI) is another advanced diffusion post-processing technique that can better resolve crossing fibers in white matter. Based on the properties of water diffusion among cellular membranes, DKI utilizes a non-Gaussian tensor model to calculate mean kurtosis (MK), axial kurtosis (AK), and radial kurtosis (RK) that more accurately assesses the movement of these molecules among tissue [47]. MK, RK, and AK indices have extended the assessment of specific biomarkers within white matter for TLE [48, 49], post-traumatic epilepsy [50], and idiopathic generalized epilepsies (IGEs) [51]. While DSI and DKI improve resolution of crossing fibers, another approach known as neurite orientation dispersion and density imaging (NODDI) [52] has recently emerged to resolve both crossing fibers and biological specificity [53, 54]. NODDI works by assessing water movement among intra- and extracellular compartments to determine the orientation and density of neurites (i.e., to any process that emanates from the soma, such as axons and dendrites). The degree to which neurites are estimated to be dispersed, condensed, or absent is then used to make voxel-wise inferences about tissue properties throughout the brain. Specifically, resulting index maps are utilized as 1) orientation dispersion index (ODI) estimates of degrees of crossing fibers or dendritic branching; 2) neurite density index (NDI), also sometimes called intracellular volume fraction (ICVF), estimate of myelination; and 3) isometric volume fraction (V-ISO) estimates of extracellular free water [55]. Although the biological specificity of NDI and ODI for indexing myelin density and axonal dispersion has been validated histologically [54, 56], such validation of edema or neuroinflammation and V-ISO remains unclear due to the active physiological nature of inflammation and methods of the histological preserving techniques [57, 58]. Further, limitations for HARDI sequences related to physical principles include increased shell size and acquisition times [59]. Notwithstanding these limitations, recent applications of NODDI analysis to neuroimaging studies of epilepsy have shown that neurite density is diminished within otherwise MRI-negative epileptic temporal lobes [60] and in focal cortical dysplasia [61]. Thus, NODDI extends diffusion tensor and spectrum modeling approaches by providing greater specificity for changes compared to standard DTI. However, potential validation of V-ISO as a marker of neuroinflammation changes in epilepsy may serve as a valuable neuroimaging development in future studies.
The glymphatic system, a central nervous system (CNS) analog to the lymphatic system, has recently received increased interest because of its potential role in brain health and disease. There is evidence that the glymphatic system provides extracellular drainage of waste within cerebrospinal fluid (CSF) and that disruption of this system may be linked to neuroinflammation and function disruption [62, 63]. Non-invasive techniques have been developed and implemented to study the glymphatic system [64]. For example, DTI-derived indices of ventricular diffusion velocity along the perpendicular planes of the projection and association fiber white matter pathways, known as the DTI-based analysis along the perivascular space (DTI-ALPS), may serve as an estimate of the glymphatic system functioning [65,66,67]. Glymphatic system activity is impaired with advanced age in disorders such as Parkison’s disease (PD) and AD [68, 69]. DTI-ALPS may provide new understanding of age-related neuroinflammatory changes in epilepsy, yet no study to date has assessed the potential unique relationship between glymphatic system activity and aging in epilepsy. However, there has been limited evidence of the validity of the DTI-ALPS method and future investigations may lend additional evidence in support of its utility.
Functional Magnetic Resonance Imaging
Functional MRI (fMRI) assesses changes in regional blood-oxygenation to determine neural function and connectivity related to information processing. Data acquired from fMRI are used to make inferences about regions or networks involved in active processing during responses to a stimulus or behaviors (task-based fMRI) or passive information processing networks during rest, in which no exogenous stimuli are presented (resting-state fMRI). The main impetus for utilizing fMRI in epilepsy has traditionally been replacing the intracarotid amobarbital procedure (IAP) used to determine hemispheric lateralization of language and verbal memory to aid surgical decisions [70]. Although traditional fMRI approaches to replace IAP perform similar in predicting post-surgical outcomes [71], recent studies have shown promise for the future utility of fMRI. For example, increased predictive validity may arise from progress in visual and verbal task-development, replication across imaging centers and platforms, evaluation of the resection of the implicated in the task brain tissue, and task-based connectivity studies [72]. Outside of pre- and post-surgical evaluations, the use of fMRI in the study of epilepsy has also contributed greatly to the understanding of focal and distributed disruptions in neural function and information processing related to seizure control, comorbidities, and other patient outcomes. Task-based fMRI epilepsy studies have shown network disruptions during psychosocial stress [1, 73], emotion recognition [74, 75], memory encoding and retrieval [76,77,78], working memory [79,80,81], language [82,83,84,85], and cognitive control tasks [86]. In one of these investigations, altered language task-related thalamic network functional connectivity is proposed as an imaging biomarker of active secondary generalization [84]. By comparing changes in cognitive performance during cognitive task paradigms, fMRI studies may also be utilized to evaluate how specific anti-seizure medications such as levetiracetam [87], topiramate [88,89,90], or cannabidiol [91, 92] affect the neural substrates of cognition in epilepsy. Thus, in addition to the growing popularity of fMRI studies in replacing IAP for surgical evaluations, this approach is also increasingly used for assessing neural basis of comorbidities and treatment outcomes. Assessment of such neural correlates may point to a common neurobiological etiology or a consequence of the seizures themselves.
While fMRI provides improvements over IAP for spatial localizations of function, the temporal resolution of fMRI does not compare to the high temporal resolution of electrophysiological assessments (EEG and MEG). By combining fMRI and EEG, studies can capitalize on the spatial benefits of fMRI and the temporal benefits of EEG. Specifically, MRI compatible EEG systems are used simultaneously with fMRI (EEG-fMRI) to provide a more granular time course assessment of event-related potentials within a specified region than would be possible with fMRI or EEG alone [93]. Recent advancements in EEG-fMRI post-processing have overcome technical challenges such as strong physiological and gradient artifacts [94]. Accordingly, improvements in assessing the concordance between EEG event-related potentials and fMRI regional brain activation will provide new insights on neurological diseases and treatment approaches [95]. Among neuroimaging studies of epilepsy, EEG-fMRI has been utilized for a variety of both clinical and research applications. For example, EEG-fMRI can be used to improve localization and surgical outcomes in MRI negative patients with focal cortical dysplasia [96] and TLE [97]. As an example, resection of the localized with EEG-fMRI putative epileptogenic region has been shown to be superior to not removing this area which was associated with poor outcome [98]. In another study, 10 out of 59 EEG-fMRI assessments provided critical information allowing progress toward resective epilepsy surgery with all 10 patients afforded good seizure outcomes at 1 year [99]. EEG-fMRI has also been extensively utilized to assess widespread and transient disruptions in language, memory, or executive function networks of IGE in order to improve decision making regarding the therapy of patients [100]. Thus, by combining EEG and fMRI imaging modalities to synthesize the advantages of each, new applications and developments in EEG-fMRI techniques offer yet another approach to uncover latent aberrant brain function that can inform treatment and better understand comorbidities in patients with epilepsy.
EEG-fMRI has been applied to generalized epilepsies as well. These studies have initially focused on investigating the fMRI correlates of generalized spike and wave discharges (GSWDs; e.g., [101,102,103]), response to medications [104], and thalamic correlates of GSWDs [105, 106]. For example, this method has previously shown that GSWDs during EEG are related to thalamic fMRI connectivity, implicating thalamocortical interactions as an important mechanism in IGEs [106, 107]. More recently, this line of questioning has been extended to better understand pre-ictal fMRI connectivity leading up to GSWD simultaneously recorded by EEG. Specifically, higher degrees of fMRI connectivity among prefrontal and motor regions in IGEs immediately preceded ictal activity, suggesting that hypersynchrony initiated from motor regions engages a prefrontal-motor network that may be a key causative factor in initiating a GSWDs [108]. Likewise, EEG-fMRI has suggested response to valproic acid may be predicted prospectively by thalamofrontal connectivity, which serves as a distinct GSWDs generator in treatment-resistant IGEs [104]. Alternatively, resting-state connectivity within the default mode network differentiates between IGEs already identified with and without treatment-resistance undergoing valproic acid regimens [109]. EEG-fMRI has also been applied to the investigation of the biological basis of symptomatic generalized epilepsies including the Lennox-Gastaut syndrome as well. For example, one study documented clear thalamic and brain stem fMRI correlates to the epileptiform discharges [110] while others investigated the generalized paroxysmal fast activity and its temporal course indicating initiation in the prefrontal cortex with later propagation to the brain stem and finally to the thalamus [111, 112].
Improvements in scanner hardware and optimization of sequences for data acquisition have led to increased temporal and spatial resolution for assessed neural functioning during task-based and resting-state fMRI studies. While beneficial to both types of fMRI studies, increases in temporal resolution via multiband echo sequences are particularly beneficial for resting-state connectivity analysis [113]. Standard correlation analysis in resting state fMRI provides assessment of the strength of functional connections from one region to another (functional connectivity). From a multi-modal perspective, combinations of structural (i.e., white matter, DTI) and functional connectivity have also demonstrated the ability to use network connectivity as a clinical tool for prediction of resective or ablative epilepsy surgery outcome [114]. Recent applications of resting-state functional connectivity suggest patients with different seizure types present unique patterns of decreased thalamocortical functional connectivity [115]. However, the latest analytical approaches have further divided and assessed static and dynamic functional connectivity during resting-state fMRI. Dynamic connectivity assesses the brain’s ability to flexibly shift between networks, while static connectivity assesses the invariant robustness of network connectivity over time [116]. Static and dynamic functional connectivity offer a more nuanced view of alterations in network connectivity in epilepsy that may help better explain seizure propagation and seizure-related disruptions. For example, static functional connectivity across the ipsilateral network diminishes in TLE over time, while dynamic functional connectivity measures show the functional independence of this ipsilateral network [117]. These findings suggest that the ipsilateral temporal lobe becomes more synchronous with the secondary generalization of the seizures and may facilitate the spread of seizures across the brain. Thus, applications of new analysis approaches that assess co-activated brain regions during resting-state fMRI has provided new models to better understand whole brain epilepsy networks.
Another class of recent advanced analysis techniques to study functional connectivity assesses directional influence, or effective connectivity (i.e., Granger causality), between brain regions [118,119,120]. In studies of epilepsy, these techniques have been utilized to assess which brain regions within a network are exerting influence on other brain regions that lead to distributed dysfunction within that network [103, 121, 122]. Accordingly, a better understanding of directional influence between regions within brain networks may aid localizing the source of network dysfunction linked to epilepsies and inform potential targets for stimulation intervention studies and treatments. Another novel method of rapid fMRI acquisition in addition to multiband echo sequences that also capitalizes on optimization of temporal resolution is magnetic resonance encephalography (MREG) [123]. Because the temporal resolution is dramatically decreased (i.e., ~ 100 ms TR for whole-brain images), MREG is capable of measuring very rapid physiological activity in the brain, such as glymphatic function related to cardiac pulsations [124]. Thus, MREG can potentially be combined with DTI-ALPS to derive better understanding of glymphatic dysfunction in epilepsy [64]. In addition, recent work has utilized MREG to demonstrate aberrant increases in cerebral pulsation rates for epilepsy patients and drug-naïve seizure patients compared to healthy controls [125]. Accordingly, improvements in scanner sequences and hardware that have increased spatial and temporal precision for fMRI sequences hold a broad spectrum of potential for assessing whole-brain functional changes associated with epilepsy.
Magnetic Resonance Spectroscopy Imaging
1H-magnetic resonance spectroscopic imaging (MRSI) is a well-established method for studying many major metabolites in the brain. Such metabolites include inhibitory neurotransmitters gamma-aminobutyric acid (GABA) or glutamate and glutamine (combined peak known as GLX), as well as N-acetyl aspartate (NAA), choline (Cho), and creatinine (Cre) serving as an index of neuronal or cellular states and metabolic function [126, 127]. Recently, the use of high field 7 T MRI has made individual metabolite assessment of glutamate (excitatory neurotransmitter) from its precursor glutamine possible, and has suggested a role of this glutamate hyperexcitability in post-stroke epileptogenesis [128]. These metabolites have traditionally been assessed in epilepsy within a single region of the brain (e.g., medial temporal or medial frontal regions) using a relatively large single-voxel, known as single-voxel MRSI. However, recent developments allow for volumetric whole-brain MRSI assessment of these metabolites [129, 130] while maintaining consistency to single-voxel approaches [131]. Applications of whole-brain MRSI to characterize distributed metabolite changes throughout the brain in epilepsy, rather than a specific region. For example, recent work examining MRSI in patients with epilepsy demonstrated widespread decreases in NAA throughout the brain including areas outside of the ictal onset zone indicating neuronal dysfunction in the absence of other signs of tissue atrophy [132, 133].
Another promising analysis advancement in the field of MRSI is the use of relative shifts in metabolite peaks to infer changes in temperature throughout the brain. Specifically, voxel-level brain temperature can be calculated using the chemical shift difference between peaks for creatine and water [134, 135]. This method, known as MRSI thermometry (MRSI-t), may provide additional assessments of focal and generalized changes in neuroinflammatory biomarkers involved associated with seizure generation and maintenance in epilepsies. Specifically, neuroinflammation has recently emerged as an important phenomenon that may be related to neuronal hyperexcitability in epilepsy [64, 136]. In general, the neuroinflammatory cascade serves as an adaptive response to repair local damage when glia activates in response to an insult, such as infection or injury [137]. However, in epilepsy, this cascade functions in the absence of tissue damage or infection and remains perpetually active which disrupts the blood–brain barrier and lowers the seizure threshold by increasing hyperexcitability throughout the brain [138, 139]. Measuring neuroinflammation of epilepsy patients in vivo currently relies on positron emission tomography (PET) imaging (see below), yet MRSI-t offers a noninvasive and efficient alternative to measure temperature and metabolic concentrations throughout the brain that is not limited by radioactive injections.
Positron Emission Tomography
Despite being an early precursor to MRI and being limited by invasive radioactive injections, important advancements have also recently occurred for PET imaging. Historically, 18Fluoro-2-deoxyglucose (18F-FDG) PET imaging of brain glucose metabolism has been a well-established and widely available technique used to localize epileptogenic foci [140]. More recently, the use of PET for the quantification of translocator protein (TSPO) density serves as another method to assess glial activation and neuroinflammation in the brain. Unlike MRSI-t, PET-TSPO has been validated as an index of glial activation over the past 25 years [141]. With the recent implications of neuroinflammation in epilepsy, PET-TSPO has become a valuable tool in understanding the role of glial activation and hyperexcitability in epilepsy. For example, recent work using PET-TSPO has demonstrated increased binding of TSPO in ipsilateral and contralateral to seizure foci temporal lobes in patients with TLE [142, 143]. PET-TSPO has also shown that although increasing dramatically following a seizure, detectable levels of TSPO during seizure-freedom may aid in surgical evaluations as well as understanding effects of seizures and epilepsy on inflammation throughout the brain [144]. Specifically, TSPO is thought to be overexpressed as part of the neuroinflammatory process [145]. Initial TSPO-PET studies using [11C]PK11195 aimed to image activated microglia; however, poor signal-to-noise ratio led to conflicting results across studies [146, 147]. The next-generation radioligand [11C]PBR28 exhibits a higher specific binding but is limited by a requirement of genetic stratification, which excludes a substantial portion of patients with epilepsy from testing [148, 149]. [11C]DPA-713, however, possesses greater specificity than [11C]PK11195 and [11C]PBR28 for discriminating between healthy and abnormal tissue. Further, [11C]DPA-713 has a much larger total volume of distribution and is less sensitive than [11C]PBR28 to the TSPO-binding polymorphism, thus increasing the proportion of patients with epilepsy that can be assessed [64].
Magnetoencephalography
MEG assesses changes in magnetic fields (i.e., dipoles) that arise from the bioelectrical signatures generated by excitatory and inhibitory postsynaptic potentials with greater precision than EEG and greater temporal resolution than fMRI [150]. EEG benefits from broader clinical utilization and research applications in studies of epilepsy due to the relative ease of implementation and greater abundance compared to MEG, which must be housed in extensive magnetic shielding. However, MEG serves as a superior electrophysiological imaging technique that extends measurement of postsynaptic potentials and provides a broader and more spatially precise assessment of the entire brain [151]. Thus, where it is available, MEG serves an important clinical tool in pre-surgical evaluations of epilepsy by detecting the unique magnetic signatures of epileptiform spikes, which aids in accurately localizing epileptogenic zones [152, 153]. In a recent study, performing MEG provided clinically useful and new information in 34% of patients undergoing presurgical testing [154]. However, the strength of the dipole signal is inversely proportional to the distance from the detector, orientation to the skull, and interferences from multiple electrical sources of epileptiform discharges in the brain [155]. Accordingly, use of invasive multimodal techniques (e.g., ECoG and sEEG) in combination with MEG is sometimes warranted for localization of epileptogenic zones. For example, MEG increases the chance of localizing an epileptic area with sEEG, while sEEG guided resection is more likely to succeed in seizure reduction when guided by positive MEG source localization [156]. Thus, MEG adds valuable precision to the presurgical evaluation by guiding placement of electrodes to improve surgical outcomes.
MEG has also recently emerged as a valuable tool in high temporal resolution whole-brain neuroimaging approaches to aberrant network function in epilepsy. For example, a recent MEG assessment of aberrant network connectivity extends the approach of fMRI network analysis by providing evidence of altered signal frequencies in TLE, including increases of delta and theta connectivity within resting-state networks [157]. Likewise, MEG findings utilizing a short temporal resolution have demonstrated increased connectivity of oscillations between brain regions in focal cortical, subcortical, and cerebellar regions during absence seizures and interictal periods [158, 159]. Thus, MEG extends current and prior fMRI approaches to assess aberrant network function in epilepsy with combined high degree of temporal and spatial resolution. This high degree of spatiotemporal resolution provides both spatial specificity and frequency information, which may be critical to epilepsy network models.
Advancements in Group-Level Analysis: Graph Theory and Machine Learning
Regardless of the neuroimaging modality, advancements of the group-level analysis methods have contributed to new understanding of how distributed networks throughout the brain are affected in epilepsy. Two relatively recent developments, graph theory analyses and machine learning, have provided particularly compelling new evidence and potential for better understanding of epilepsy as a network disorder.
Graph Theory
Graph theory metrics have increased our understanding of how brain networks are disrupted in epilepsy. To simplify several of the high-level mathematical concepts that comprise fundamental metrics of graph theory [160], such as centrality, small-worldness, and global efficiency, consider airport travel as a relatable model of transmission within a network. In order for passengers to travel from origin to destination, connecting flights between airports in this network must be made. Centrality, in this example, is an index of how many travel connections rely on a given airport of the network. Because there are a higher number of total flights made in or out of the Atlanta Hartsfield (ATL) airport, GA than the John F. Kennedy (JFK) airport, NY, the importance (or centrality) for the network is higher for ATL than for JFK. Thus, both of these airports form part of a network, yet they have different centralities or importance for the cohesiveness of this network. Alternatively, other graph theory metrics are a function of the entire network itself, such as small-world connectivity. For this example, imagine that instead of comparing airports to nodes of a network, we are comparing two different airlines (i.e., networks). Small-worldness, in this analogy, is scaled as a function of whether some airports connect mostly with smaller sub-networks that contain few connections to outside airports (high small-worldness), or whether connections are equally distributed across all airports (low small-worldness). Because Airline A has many flights with a terminal destination in the southeast that will connect through ATL (i.e., a module of hubs), while many flights with a terminal destination in the northeast will connect through JFK (i.e., another module of hubs), the modularity, or small-worldness, would be higher for Airline A. However, if Airline B offers many direct-flights to final destinations without connecting flights, the small-worldness for Airline B would be relatively low (and likely with very high in travel costs). Another example is global efficiency, which assesses the shortness of path length and connections within a network. Airline A most frequently offers tickets that will require the passenger to make two connecting flights with a total flight time of 11 h, while Airline B most frequently offers tickets with a direct-flight and a total flight time of only 2 h. Airline A, in this example, would be less efficient than Airline B based on the total number of flights and duration of the air travel. Application of graph theory metrics, as exemplified above, has brought a new approach to characterizing and assessing complex neural networks.
By applying graph theory analysis to characterize neural networks in epilepsy, resting-state connectivity and white matter tractography analyses have been used separately and sometimes combined [2]. Recent findings have shown changes in ipsilateral node centrality associated with TLE [161, 162]. Likewise, decreases in global efficiency and small-worldness are associated with both generalized [163] and focal [164, 165] epilepsies. Furthermore, centrality, small-worldness, and global efficiency measures have also shown early promise in the utility to predict surgical outcomes in anterior temporal lobectomy [166,167,168]. Accordingly, innovative applications of graph theory assessments to neuroimaging analyses provide a valuable extension to prior assessments and a more sophisticated characterization of whole-brain neural networks that will broaden our understanding of epilepsy as a network disorder.
Machine Learning Classifiers
Machine learning, as applied to neuroimaging, is a class of artificial intelligence methods that attempt to train computer-generated algorithms to cluster or classify sets of neuroimaging data [169]. Of particular interest to clinical diagnoses and research applications in epilepsy, machine learning classifiers hold significant potential for utilizing neuroimaging data to identify biomarkers of neurological and psychiatric disease states [170]. Within new emerging studies of epilepsy networks, machine learning techniques play an important role in identifying aberrant brain networks across the gamut of imaging modalities and can serve to aid in predicting surgical outcomes and future diagnostic and seizure treatment approaches [171]. For example, unsupervised machine learning, which in contrast to supervised machine learning that includes a clinical diagnosis to train models, bypasses the notion of an a priori categorization and instead allows a data driven model to cluster datasets based on like and unlike components [172]. Unsupervised machine learning classifiers hold the potential for identifying distributed components of epileptic disorders forming patterns that were not previously understood, such as predictors of surgical outcomes [173], variability in dysplasia subtypes [29], and improvements in lesion detection via morphometric MRI post-processing (MAP) of MRI-negative patients [23, 174]. From a clinical and research perspective, increased widespread utilization and familiarity with machine learning techniques applied to whole-brain neuroimaging assessments will provide vital new understanding of epilepsy as a network disorder.
Imaging Mental Health Comorbidities in Epilepsies
In this review, we have so far highlighted some of the promising recent developments and advancements in human neuroimaging. In this section, we will discuss important future directions for these neuroimaging studies with two points of emphasis. First, from a neurotherapeutic perspective, assessment of the neurobiological basis of the bidirectional relationship between epilepsy and mental health comorbidities (i.e., mood, anxiety) can provide a better understanding of the functional network alterations associated with epilepsy and involved in seizure precipitates. Second, although temporal lobe epilepsy is at the forefront of human epilepsy research and should continue to be studied extensively, idiopathic generalized epilepsies (IGEs) and extra-temporal neocortical epilepsies (e.g., frontal lobe epilepsies) are under-investigated but can serve as valuable models for understanding both epilepsy and psychiatric conditions. Despite the synchrony and episodic nature of both mental health disorders and epilepsy [175], there is a paucity of studies that focus on their corresponding fluctuations [176].
Mental Health Comorbidities
The bidirectional relationship between mental health comorbidities and epilepsy constitutes a major health concern. Of the 70 million people with epilepsy worldwide [177], nearly 50% will develop mental health comorbidities and people with psychiatric disorders are more likely to develop epilepsy [7, 178]. Given that mood and anxiety disorders carry nearly a 30% lifetime prevalence rate [179], the need for better understanding of the neurobiological mechanisms that underlie the bidirectional relationship between mental health comorbidities and epilepsies extends far beyond the treatment of epilepsy alone. In fact, investigating the interplay between mental health comorbidities and epilepsies will provide guidance on how mental health symptom improvement impacts response to epilepsy treatment, and vice versa. Further, understanding the link between mental health symptoms and seizure control may shape the clinical management and future treatment studies of both entities.
The relationship between epilepsy and mental health comorbidities may arise from disruptions in the prefrontal cortex-hippocampal-amygdala (PFC-HC-AMY) network that produces symptoms of depression and anxiety due to a large degree of overlapping changes within these regions and their connectivity [8, 180]. In general, patients with poorly controlled epilepsy can predict subsequent seizure occurrence immediately after acute stress experience [181,182,183]. Behavioral stress management techniques like progressive muscle relaxation, biofeedback, and cognitive-behavioral therapy have been proposed as adjunct to seizure medications [184, 185] with their efficacy depending on whether they reduce perceived stress [186, 187]. Thus, an important area of future research is better understanding of this bidirectional relationship between mood, stress, and epilepsy. TLEs have a high rate of comorbid depression and anxiety [188] and are associated with disruption within the PFC-HC-AMY circuit underlying emotion processes [1, 73, 74, 183, 189]. Furthermore, psychiatric comorbidities and cognitive dysfunction in TLE are associated with volumetric AMY increases [190] and hippocampal sclerosis [191], which is common among TLEs but not necessarily mental health comorbidities. Consequently, abnormalities in neural transmission originating within HC-AMY regions induce broadly distributed network disruption, including PFC connectivity [176]. Extending the focus beyond discrete HC and AMY abnormalities underlying mental health comorbidities and epilepsy to fronto-limbic network dysfunction is consistent with recent shifts in emphasis from regional to network models among mental health comorbidities [3, 192]. Thus, a better understanding of disruptions in specific neural networks related to epilepsies and impaired inhibition of emotion responses will serve as a valuable model for better understanding of epilepsy as a network disorder.
Mental Health Comorbidities in Generalized Epilepsies
The vast majority of prior literature on the relationship between mental health comorbidities and epilepsies has assessed a locus of deficit arising from a specific epileptic focus (i.e., TLEs). Furthermore, TLEs typically have a relatively poor prognosis for treatment response [193]. Thus, TLEs may have limited potential as a model for assessing neurobiology linked to mental health comorbidities given a poor treatment response among HC-AMY structural abnormalities and mental health symptom severity. IGEs constitute ~ 20% of all epilepsy diagnoses and include patients with absence, myoclonic, and generalized tonic–clonic seizures [194]. As IGEs are non-focal, seizure onset occurs over a large cortical and subcortical network that includes a thalamic hub, rather than a specific node, with rapid spread via a network of cortical and subcortical regions [195]. Thus, IGEs are classified by widespread cortical spike-and-wave discharges and seizures without a definite focal origin [105, 196]. Consequently, comorbid mental health pathophysiology in IGEs extends beyond the scope of disruptions in function and connectivity that arise from a specific epileptic focus [197, 198]. Mental health comorbidities are estimated to occur in up to 50% of adult patients with IGEs. Yet, IGEs have a relatively good prognosis and high remission rate with treatment (64–82%) [193]. IGEs are associated with decreased functional connectivity within medial PFC and limbic regions [109, 199,200,201], which correlates with increased seizure frequency [199]. Interestingly, dMRI studies have shown degradation within the uncinate fasciculus (PFC-AMY) and fornix (HC-hypothalamus) white matter pathways in patients with IGEs [202]. Further, IGEs are associated with broad disruptions in frontal lobe function and connectivity that may specifically affect emotion processes and inhibitory control that generalize broadly to depressive and anxiety mental health comorbidities [203, 204]. Despite the vast potential for utilizing IGEs as a model to understand changes in neural networks related to mental health comorbidity status, underlying links between IGEs and mental health comorbidities have received modest attention compared to vastly more commonly studied TLEs. Future research investigating disruptions of specific neural networks related to fluctuations in mental health states in IGE will serve as a valuable model for better understanding epilepsy as a network disorder.
Conclusion
Recent advancements in neuroimaging methods, including MRI, PET, MEG, and techniques discussed in this review such as graph theory and machine learning have opened new potential for better understanding of epilepsy as a network disorder. The techniques and methods reviewed are of particular importance for network models of epilepsy given they provide a variety of functional and structural measurements at a whole-brain global level. Utilizing these advanced techniques to assess mental health comorbidities in IGEs will drive forward the understanding of epilepsy as a network disorder. IGEs are an ideal model to assess particular changes in emotion processing and brain network connectivity that correspond to changes in comorbid mental health symptoms given that they have relatively high comorbidity and remission rates, and network pathophysiology that does not arise from a specific epileptic focus.
References
Goodman AM, Allendorfer JB, Heyse H, Szaflarski BA, Eliassen JC, Nelson EB, et al. Neural response to stress and perceived stress differ in patients with left temporal lobe epilepsy. Hum Brain Mapp. 2019;40:3415-3430.
Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: The emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 2015;50:162-170.
Bassett DS, Xia CH, Satterthwaite TD. Understanding the Emergence of Neuropsychiatric Disorders With Network Neuroscience. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:742-753.
Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9:3003.
Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry. 2015;77:223-235.
Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472-480.
Goldman AM, LaFrance WC, Jr, Benke T, Asato M, Drane D, Pack A, et al. 2014 Epilepsy Benchmarks Area IV: Limit or Prevent Adverse Consequence of Seizures and Their Treatment Across The Lifespan. Epilepsy Curr. 2016;16:198-205.
Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, et al. Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24:156-168.
Middlebrooks EH, Ver Hoef L, Szaflarski JP. Neuroimaging in Epilepsy. Curr Neurol Neurosci Rep. 2017;17:32.
Crosson B, Ford A, McGregor KM, Meinzer M, Cheshkov S, Li X, et al. Functional imaging and related techniques: an introduction for rehabilitation researchers. J Rehabil Res Dev. 2010;47:vii-xxxiv.
Ho K, Lawn N, Bynevelt M, Lee J, Dunne J. Neuroimaging of first-ever seizure: Contribution of MRI if CT is normal. Neurol Clin Pract. 2013;3:398-403.
Grant PE. Structural MR imaging. Epilepsia. 2004;45 Suppl 4:4-16.
Coan AC, Kubota B, Bergo FP, Campos BM, Cendes F. 3T MRI quantification of hippocampal volume and signal in mesial temporal lobe epilepsy improves detection of hippocampal sclerosis. AJNR Am J Neuroradiol. 2014;35:77-83.
Ver Hoef LW, Williams FB, Kennedy RE, Szaflarski JP, Knowlton RC. Predictive value of hippocampal internal architecture asymmetry in temporal lobe epilepsy. Epilepsy Res. 2013;106:155-163.
Ver Hoef LW, Paige AL, Riley KO, Cure J, Soltani M, Williams FB, et al. Evaluating hippocampal internal architecture on MRI: inter-rater reliability of a proposed scoring system. Epilepsy Res. 2013;106:146-154.
Fleming Beattie J, Martin RC, Kana RK, Deshpande H, Lee S, Cure J, et al. Hippocampal dentation: Structural variation and its association with episodic memory in healthy adults. Neuropsychologia. 2017;101:65-75.
Ver Hoef L, Deshpande H, Cure J, Selladurai G, Beattie J, Kennedy RE, et al. Clear and Consistent Imaging of Hippocampal Internal Architecture With High Resolution Multiple Image Co-registration and Averaging (HR-MICRA). Front Neurosci. 2021;15:546312.
Chang C, Huang C, Zhou N, Li SX, Ver Hoef L, Gao Y. The bumps under the hippocampus. Hum Brain Mapp. 2018;39:472-490.
Wisse LE, Kuijf HJ, Honingh AM, Wang H, Pluta JB, Das SR, et al. Automated Hippocampal Subfield Segmentation at 7T MRI. AJNR Am J Neuroradiol. 2016;37:1050-1057.
Zhang Y, Lv Y, You H, Dou W, Hou B, Shi L, et al. Study of the hippocampal internal architecture in temporal lobe epilepsy using 7T and 3T MRI. Seizure. 2019;71:116-123.
De Ciantis A, Barba C, Tassi L, Cosottini M, Tosetti M, Costagli M, et al. 7T MRI in focal epilepsy with unrevealing conventional field strength imaging. Epilepsia. 2016;57:445-454.
Wang I, Oh S, Blumcke I, Coras R, Krishnan B, Kim S, et al. Value of 7T MRI and post-processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia. 2020;61:2509-2520.
Wang ZI, Jones SE, Jaisani Z, Najm IM, Prayson RA, Burgess RC, et al. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol. 2015;77:1060-1075.
Wang S, Jin B, Aung T, Katagiri M, Jones SE, Krishnan B, et al. Application of MRI Post-processing in Presurgical Evaluation of Non-lesional Cingulate Epilepsy. Front Neurol. 2018;9:1013.
Mintzer S. A MAP of Seizure-Freedom in Patients with a Normal MRI Scan. Epilepsy Curr. 2016;16:94-95.
Wagner J, Weber B, Urbach H, Elger CE, Huppertz HJ. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain. 2011;134:2844-2854.
Jaisani Z, Riley K, Ver Hoef L, Szaflarski JP, editors. Pilot data from first prospective study utilizing voxel based morphometry to identify epileptogenic lesion in MRI negative refractory epilepsy patients. Annual Meeting of the American Epilepsy Society; 2019 12/2019; Baltimore, MD, USA.
Hong SJ, Kim H, Schrader D, Bernasconi N, Bernhardt BC, Bernasconi A. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014;83:48-55.
Lee HM, Gill RS, Fadaie F, Cho KH, Guiot MC, Hong SJ, et al. Unsupervised machine learning reveals lesional variability in focal cortical dysplasia at mesoscopic scale. Neuroimage Clin. 2020;28:102438.
Garbelli R, Milesi G, Medici V, Villani F, Didato G, Deleo F, et al. Blurring in patients with temporal lobe epilepsy: clinical, high-field imaging and ultrastructural study. Brain. 2012;135:2337-2349.
Ladd ME, Bachert P, Meyerspeer M, Moser E, Nagel AM, Norris DG, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1-50.
Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, et al. Magnetic resonance fingerprinting. Nature. 2013;495:187-192.
Bernasconi N, Wang I. Emerging Trends in Neuroimaging of Epilepsy. Epilepsy Curr. 2021:1535759721991161.
Liao C, Wang K, Cao X, Li Y, Wu D, Ye H, et al. Detection of Lesions in Mesial Temporal Lobe Epilepsy by Using MR Fingerprinting. Radiology. 2018;288:804-812.
Wang K, Cao X, Wu D, Liao C, Zhang J, Ji C, et al. Magnetic resonance fingerprinting of temporal lobe white matter in mesial temporal lobe epilepsy. Ann Clin Transl Neurol. 2019;6:1639-1646.
Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209-219.
Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140.
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714-1722.
Wheeler-Kingshott CA, Ciccarelli O, Schneider T, Alexander DC, Cercignani M. A new approach to structural integrity assessment based on axial and radial diffusivities. Funct Neurol. 2012;27:85-90.
Hatton SN, Huynh KH, Bonilha L, Abela E, Alhusaini S, Altmann A, et al. White matter abnormalities across different epilepsy syndromes in adults: an ENIGMA-Epilepsy study. Brain. 2020;143:2454-2473.
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316-329.
Budde MD, Annese J. Quantification of anisotropy and fiber orientation in human brain histological sections. Front Integr Neurosci. 2013;7:3.
Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front Neurol. 2018;9:92.
Keller SS, Glenn GR, Weber B, Kreilkamp BA, Jensen JH, Helpern JA, et al. Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy. Brain. 2017;140:68-82.
Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267-1277.
Chen CL, Shih YC, Liou HH, Hsu YC, Lin FH, Tseng WI. Premature white matter aging in patients with right mesial temporal lobe epilepsy: A machine learning approach based on diffusion MRI data. Neuroimage Clin. 2019;24:102033.
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53:1432-1440.
Bonilha L, Lee CY, Jensen JH, Tabesh A, Spampinato MV, Edwards JC, et al. Altered microstructure in temporal lobe epilepsy: a diffusional kurtosis imaging study. AJNR Am J Neuroradiol. 2015;36:719-724.
Lemkaddem A, Daducci A, Kunz N, Lazeyras F, Seeck M, Thiran JP, et al. Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. Neuroimage Clin. 2014;5:349-358.
Li W, Wang X, Wei X, Wang M. Susceptibility-weighted and diffusion kurtosis imaging to evaluate encephalomalacia with epilepsy after traumatic brain injury. Ann Clin Transl Neurol. 2018;5:552-558.
Lee CY, Tabesh A, Spampinato MV, Helpern JA, Jensen JH, Bonilha L. Diffusional kurtosis imaging reveals a distinctive pattern of microstructural alternations in idiopathic generalized epilepsy. Acta Neurol Scand. 2014;130:148-155.
Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000-1016.
Edwards LJ, Pine KJ, Ellerbrock I, Weiskopf N, Mohammadi S. NODDI-DTI: Estimating Neurite Orientation and Dispersion Parameters from a Diffusion Tensor in Healthy White Matter. Front Neurosci. 2017;11:720.
Sato K, Kerever A, Kamagata K, Tsuruta K, Irie R, Tagawa K, et al. Understanding microstructure of the brain by comparison of neurite orientation dispersion and density imaging (NODDI) with transparent mouse brain. Acta Radiol Open. 2017;6:2058460117703816.
Goodman AM, Allendorfer JB, Blum AS, Bolding MS, Correia S, Ver Hoef LW, et al. White matter and neurite morphology differ in psychogenic nonepileptic seizures. Ann Clin Transl Neurol. 2020;7:1973-1984.
Sepehrband F, Clark KA, Ullmann JF, Kurniawan ND, Leanage G, Reutens DC, et al. Brain tissue compartment density estimated using diffusion-weighted MRI yields tissue parameters consistent with histology. Hum Brain Mapp. 2015;36:3687-3702.
Grussu F, Schneider T, Tur C, Yates RL, Tachrount M, Ianus A, et al. Neurite dispersion: a new marker of multiple sclerosis spinal cord pathology? Ann Clin Transl Neurol. 2017;4:663-679.
Palacios EM, Owen JP, Yuh EL, Wang MB, Vassar MJ, Ferguson AR, et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci Adv. 2020;6:eaaz6892.
Descoteaux M. High Angular Resolution Diffusion Imaging (HARDI). Wiley Encyclopedia of Electrical and Electronics Engineering. p. 1–25.
Sone D, Sato N, Ota M, Maikusa N, Kimura Y, Matsuda H. Abnormal neurite density and orientation dispersion in unilateral temporal lobe epilepsy detected by advanced diffusion imaging. Neuroimage Clin. 2018;20:772-782.
Winston GP, Micallef C, Symms MR, Alexander DC, Duncan JS, Zhang H. Advanced diffusion imaging sequences could aid assessing patients with focal cortical dysplasia and epilepsy. Epilepsy Res. 2014;108:336-339.
Plog BA, Nedergaard M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu Rev Pathol. 2018;13:379-394.
Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology. 2019;65:106-119.
Sharma AA, Szaflarski JP. In vivo Imaging of Neuroinflammatory Targets in Treatment-Resistant Epilepsy. Curr Neurol Neurosci Rep. 2020;20:5.
Taoka T, Naganawa S. Glymphatic imaging using MRI. J Magn Reson Imaging. 2020;51:11-24.
Taoka T, Naganawa S. Neurofluid Dynamics and the Glymphatic System: A Neuroimaging Perspective. Korean J Radiol. 2020;21:1199-1209.
Yokota H, Vijayasarathi A, Cekic M, Hirata Y, Linetsky M, Ho M, et al. Diagnostic Performance of Glymphatic System Evaluation Using Diffusion Tensor Imaging in Idiopathic Normal Pressure Hydrocephalus and Mimickers. Curr Gerontol Geriatr Res. 2019;5675014.
Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35:172-178.
Sundaram S, Hughes RL, Peterson E, Muller-Oehring EM, Bronte-Stewart HM, Poston KL, et al. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson's disease. Neurosci Biobehav Rev. 2019;103:305-315.
Szaflarski JP, Gloss D, Binder JR, Gaillard WD, Golby AJ, Holland SK, et al. Practice guideline summary: Use of fMRI in the presurgical evaluation of patients with epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017;88:395-402.
Bauer PR, Reitsma JB, Houweling BM, Ferrier CH, Ramsey NF. Can fMRI safely replace the Wada test for preoperative assessment of language lateralisation? A meta-analysis and systematic review. J Neurol Neurosurg Psychiatry. 2014;85:581-588.
Szaflarski JP. Is fMRI Ready to Replace IAP? Wait, Wait,...We Are Not There Yet! Epilepsy Curr. 2020;20(suppl 6):6S-8S.
Allendorfer JB, Heyse H, Mendoza L, Nelson EB, Eliassen JC, Storrs JM, et al. Physiologic and cortical response to acute psychosocial stress in left temporal lobe epilepsy - a pilot cross-sectional fMRI study. Epilepsy Behav. 2014;36:115-123.
Szaflarski JP, Allendorfer JB, Heyse H, Mendoza L, Szaflarski BA, Cohen N. Functional MRI of facial emotion processing in left temporal lobe epilepsy. Epilepsy Behav. 2014;32:92-99.
Morningstar M, Hung A, Grannis C, French RC, Mattson WI, Ostendorf AP, et al. Blunted neural response to emotional faces in the fusiform and superior temporal gyrus may be marker of emotion recognition deficits in pediatric epilepsy. Epilepsy Behav. 2020;112:107432.
Hill PF, King DR, Lega BC, Rugg MD. Comparison of fMRI correlates of successful episodic memory encoding in temporal lobe epilepsy patients and healthy controls. Neuroimage. 2020;207:116397.
Sidhu MK, Stretton J, Winston GP, Symms M, Thompson PJ, Koepp MJ, et al. Factors affecting reorganisation of memory encoding networks in temporal lobe epilepsy. Epilepsy Res. 2015;110:1-9.
Sidhu MK, Stretton J, Winston GP, Bonelli S, Centeno M, Vollmar C, et al. A functional magnetic resonance imaging study mapping the episodic memory encoding network in temporal lobe epilepsy. Brain. 2013;136:1868-1888.
Nair S, Szaflarski JP. Neuroimaging of memory in frontal lobe epilepsy. Epilepsy Behav. 2020;103:106857.
Braakman HM, Vaessen MJ, Jansen JF, Debeij-van Hall MH, de Louw A, Hofman PA, et al. Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy. Epilepsia. 2013;54:446-454.
Stretton J, Sidhu MK, Winston GP, Bartlett P, McEvoy AW, Symms MR, et al. Working memory network plasticity after anterior temporal lobe resection: a longitudinal functional magnetic resonance imaging study. Brain. 2014;137:1439-1453.
Trimmel K, Caciagli L, Xiao F, van Graan LA, Koepp MJ, Thompson PJ, et al. Impaired naming performance in temporal lobe epilepsy: language fMRI responses are modulated by disease characteristics. J Neurol. 2020.
Kaestner E, Reyes A, Macari AC, Chang YH, Paul BM, Hermann BP, et al. Identifying the neural basis of a language-impaired phenotype of temporal lobe epilepsy. Epilepsia. 2019;60:1627-1638.
Caciagli L, Allen LA, He X, Trimmel K, Vos SB, Centeno M, et al. Thalamus and focal to bilateral seizures: A multiscale cognitive imaging study. Neurology. 2020;95:e2427-e2441.
Croft LJ, Baldeweg T, Sepeta L, Zimmaro L, Berl MM, Gaillard WD. Vulnerability of the ventral language network in children with focal epilepsy. Brain. 2014;137:2245-2257.
Guo L, Bai G, Zhang H, Lu D, Zheng J, Xu G. Cognitive Functioning in Temporal Lobe Epilepsy: A BOLD-fMRI Study. Mol Neurobiol. 2017;54:8361-8369.
Wandschneider B, Stretton J, Sidhu M, Centeno M, Kozak LR, Symms M, et al. Levetiracetam reduces abnormal network activations in temporal lobe epilepsy. Neurology. 2014;83:1508-1512.
Wandschneider B, Burdett J, Townsend L, Hill A, Thompson PJ, Duncan JS, et al. Effect of topiramate and zonisamide on fMRI cognitive networks. Neurology. 2017;88:1165-1171.
Yasuda CL, Centeno M, Vollmar C, Stretton J, Symms M, Cendes F, et al. The effect of topiramate on cognitive fMRI. Epilepsy Res. 2013;105:250-255.
Szaflarski JP, Allendorfer JB. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav. 2012;24:74-80.
Allendorfer JB, Nenert R, Bebin EM, Gaston TE, Grayson LE, Hernando KA, et al. fMRI study of cannabidiol-induced changes in attention control in treatment-resistant epilepsy. Epilepsy Behav. 2019;96:114-121.
Gaston TE, Allendorfer JB, Nair S, Bebin EM, Grayson LP, Martin RC, et al. Effects of highly purified cannabidiol (CBD) on fMRI of working memory in treatment-resistant epilepsy. Epilepsy Behav. 2020;112:107358.
Debener S, Ullsperger M, Siegel M, Engel AK. Single-trial EEG-fMRI reveals the dynamics of cognitive function. Trends Cogn Sci. 2006;10:558-563.
Marino M, Liu Q, Koudelka V, Porcaro C, Hlinka J, Wenderoth N, et al. Adaptive optimal basis set for BCG artifact removal in simultaneous EEG-fMRI. Sci Rep. 2018;8:8902.
Mele G, Cavaliere C, Alfano V, Orsini M, Salvatore M, Aiello M. Simultaneous EEG-fMRI for Functional Neurological Assessment. Front Neurol. 2019;10:848.
Pittau F, Ferri L, Fahoum F, Dubeau F, Gotman J. Contributions of EEG-fMRI to Assessing the Epileptogenicity of Focal Cortical Dysplasia. Front Comput Neurosci. 2017;11:8.
Coan AC, Chaudhary UJ, Grouiller F, Campos BM, Perani S, De Ciantis A, et al. EEG-fMRI in the presurgical evaluation of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2016;87:642-649.
An D, Fahoum F, Hall J, Olivier A, Gotman J, Dubeau F. Electroencephalography/functional magnetic resonance imaging responses help predict surgical outcome in focal epilepsy. Epilepsia. 2013;54:2184-2194.
Kowalczyk MA, Omidvarnia A, Abbott DF, Tailby C, Vaughan DN, Jackson GD. Clinical benefit of presurgical EEG-fMRI in difficult-to-localize focal epilepsy: A single-institution retrospective review. Epilepsia. 2020;61:49-60.
Shamshiri EA, Sheybani L, Vulliemoz S. The Role of EEG-fMRI in Studying Cognitive Network Alterations in Epilepsy. Front Neurol. 2019;10:1033.
Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. J Neurosci. 2010;30:5884-5893.
Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, et al. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127-1144.
Szaflarski JP, DiFrancesco M, Hirschauer T, Banks C, Privitera MD, Gotman J, et al. Cortical and subcortical contributions to absence seizure onset examined with EEG/fMRI. Epilepsy Behav. 2010;18:404-413.
Szaflarski JP, Kay B, Gotman J, Privitera MD, Holland SK. The relationship between the localization of the generalized spike and wave discharge generators and the response to valproate. Epilepsia. 2013;54:471-480.
Kay B, Szaflarski JP. EEG/fMRI contributions to our understanding of genetic generalized epilepsies. Epilepsy Behav. 2014;34:129-135.
Tyvaert L, Chassagnon S, Sadikot A, LeVan P, Dubeau F, Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology. 2009;73:2018-2022.
Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, et al. EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31(4):1700-1710.
Tangwiriyasakul C, Perani S, Centeno M, Yaakub SN, Abela E, Carmichael DW, et al. Dynamic brain network states in human generalized spike-wave discharges. Brain. 2018;141:2981-2994.
Kay BP, DiFrancesco MW, Privitera MD, Gotman J, Holland SK, Szaflarski JP. Reduced default mode network connectivity in treatment-resistant idiopathic generalized epilepsy. Epilepsia. 2013;54:461-470.
Siniatchkin M, Coropceanu D, Moeller F, Boor R, Stephani U. EEG-fMRI reveals activation of brainstem and thalamus in patients with Lennox-Gastaut syndrome. Epilepsia. 2011;52:766-774.
Archer JS, Warren AE, Stagnitti MR, Masterton RA, Abbott DF, Jackson GD. Lennox-Gastaut syndrome and phenotype: secondary network epilepsies. Epilepsia. 2014;55:1245-1254.
Warren AEL, Harvey AS, Vogrin SJ, Bailey C, Davidson A, Jackson GD, et al. The epileptic network of Lennox-Gastaut syndrome: Cortically driven and reproducible across age. Neurology. 2019;93:e215-e226.
Demetriou L, Kowalczyk OS, Tyson G, Bello T, Newbould RD, Wall MB. A comprehensive evaluation of increasing temporal resolution with multiband-accelerated protocols and effects on statistical outcome measures in fMRI. Neuroimage. 2018;176:404-416.
Morgan VL, Englot DJ, Rogers BP, Landman BA, Cakir A, Abou-Khalil BW, et al. Magnetic resonance imaging connectivity for the prediction of seizure outcome in temporal lobe epilepsy. Epilepsia. 2017;58:1251-1260.
He X, Doucet GE, Sperling M, Sharan A, Tracy JI. Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia. 2015;56:1571-1579.
Rangaprakash D, Deshpande G, Daniel TA, Goodman AM, Robinson JL, Salibi N, et al. Compromised hippocampus-striatum pathway as a potential imaging biomarker of mild-traumatic brain injury and posttraumatic stress disorder. Hum Brain Mapp. 2017;38:2843-2864.
Morgan VL, Abou-Khalil B, Rogers BP. Evolution of functional connectivity of brain networks and their dynamic interaction in temporal lobe epilepsy. Brain Connect. 2015;5(1):35-44.
Deshpande G, Sathian K, Hu X. Assessing and compensating for zero-lag correlation effects in time-lagged granger causality analysis of fMRI. IEEE Transactions on Biomedical Engineering. 2010;57(6):1446-1456.
Deshpande G, Jia H. Multi-Level Clustering of Dynamic Directional Brain Network Patterns and Their Behavioral Relevance. Frontiers in Neuroscience. 2020;13(1448).
Nenert R, Allendorfer JB, Szaflarski JP. A model for visual memory encoding. PLoS One. 2014;9:e107761.
Wei H, An J, Shen H, Zeng LL, Qiu S, Hu D. Altered Effective Connectivity among Core Neurocognitive Networks in Idiopathic Generalized Epilepsy: An fMRI Evidence. Frontiers in human neuroscience. 2016;10:447.
Jiang LW, Qian RB, Fu XM, Zhang D, Peng N, Niu CS, et al. Altered attention networks and DMN in refractory epilepsy: A resting-state functional and causal connectivity study. Epilepsy Behav. 2018;88:81-86.
Hennig J, Zhong K, Speck O. MR-Encephalography: Fast multi-channel monitoring of brain physiology with magnetic resonance. Neuroimage. 2007;34:212-219.
Kiviniemi V, Wang X, Korhonen V, Keinanen T, Tuovinen T, Autio J, et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab. 2016;36:1033-1045.
Kananen J, Helakari H, Korhonen V, Huotari N, Jarvela M, Raitamaa L, et al. Respiratory-related brain pulsations are increased in epilepsy-a two-centre functional MRI study. Brain Commun. 2020;2:fcaa076.
Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270:658-679.
Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo brain concentrations of N-acetyl compounds, creatine, and choline in Alzheimer disease. Arch Gen Psychiatry. 1999;56:185-192.
Nicolo JP, O'Brien TJ, Kwan P. Role of cerebral glutamate in post-stroke epileptogenesis. Neuroimage Clin. 2019;24:102069.
Maudsley AA, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, et al. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn Reson Med. 2009;61:548-559.
Maudsley AA, Domenig C, Sheriff S. Reproducibility of serial whole-brain MR spectroscopic imaging. NMR Biomed. 2010;23:251-256.
Zhang Y, Taub E, Salibi N, Uswatte G, Maudsley AA, Sheriff S, et al. Comparison of reproducibility of single voxel spectroscopy and whole-brain magnetic resonance spectroscopy imaging at 3T. NMR Biomed. 2018;31:e3898.
Kirov, II, Kuzniecky R, Hetherington HP, Soher BJ, Davitz MS, Babb JS, et al. Whole brain neuronal abnormalities in focal epilepsy quantified with proton MR spectroscopy. Epilepsy Res. 2018;139:85-91.
Maudsley AA, Domenig C, Ramsay RE, Bowen BC. Application of volumetric MR spectroscopic imaging for localization of neocortical epilepsy. Epilepsy Res. 2010;88:127-138.
Maudsley AA, Goryawala MZ, Sheriff S. Effects of tissue susceptibility on brain temperature mapping. Neuroimage. 2017;146:1093-1101.
Sharma AA, Nenert R, Mueller C, Maudsley AA, Younger JW, Szaflarski JP. Repeatability and Reproducibility of in-vivo Brain Temperature Measurements. Frontiers in human neuroscience. 2020;14:598435.
Koepp MJ, Arstad E, Bankstahl JP, Dedeurwaerdere S, Friedman A, Potschka H, et al. Neuroinflammation imaging markers for epileptogenesis. Epilepsia. 2017;58 Suppl 3:11-19.
DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139 Suppl 2:136-53.
Bascunana P, Gendron T, Sander K, Jahreis I, Polyak A, Ross TL, et al. Ex vivo characterization of neuroinflammatory and neuroreceptor changes during epileptogenesis using candidate positron emission tomography biomarkers. Epilepsia. 2019;60:2325-2333.
Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15:459-472.
Sarikaya I. PET studies in epilepsy. Am J Nucl Med Mol Imaging. 2015;5:416-430.
Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A, et al. The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans. 2015;43(4):586-592.
Gershen LD, Zanotti-Fregonara P, Dustin IH, Liow JS, Hirvonen J, Kreisl WC, et al. Neuroinflammation in Temporal Lobe Epilepsy Measured Using Positron Emission Tomographic Imaging of Translocator Protein. JAMA Neurol. 2015;72:882-888.
Hirvonen J, Kreisl WC, Fujita M, Dustin I, Khan O, Appel S, et al. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med. 2012;53:234-240.
Butler T, Li Y, Tsui W, Friedman D, Maoz A, Wang X, et al. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia. 2016;57:e191-194.
Vivash L, O'Brien TJ. Imaging Microglial Activation with TSPO PET: Lighting Up Neurologic Diseases? J Nucl Med. 2016;57:165-168.
Banati RB, Goerres GW, Myers R, Gunn RN, Turkheimer FE, Kreutzberg GW, et al. [11C](R)-PK11195 positron emission tomography imaging of activated microglia in vivo in Rasmussen's encephalitis. Neurology. 1999;53:2199-203.
Butler T, Ichise M, Teich AF, Gerard E, Osborne J, French J, et al. Imaging inflammation in a patient with epilepsy due to focal cortical dysplasia. J Neuroimaging. 2013;23:129-131.
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1-5.
Sharma AA, Szaflarski JP. Seizing the Neuroinflammatory Target: The Quest Continues. Epilepsy Curr. 2019;19:379-381.
Proudfoot M, Woolrich MW, Nobre AC, Turner MR. Magnetoencephalography. Practical Neurology. 2014;14:336-343.
Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron. 2013;80:1112–1128.
Kharkar S, Knowlton R. Magnetoencephalography in the presurgical evaluation of epilepsy. Epilepsy Behav. 2015;46:19-26.
Yin C, Zhang X, Chen Z, Li X, Wu S, Lv P, et al. Detection and localization of interictal ripples with magnetoencephalography in the presurgical evaluation of drug-resistant insular epilepsy. Brain Res. 2018.
Duez L, Tankisi H, Hansen PO, Sidenius P, Sabers A, Pinborg LH, et al. Electromagnetic source imaging in presurgical workup of patients with epilepsy: A prospective study. Neurology. 2019;92:e576-e586.
Szaflarski JP. Magnetoencephalography and Stereo-EEG Unite! Epilepsy Curr. 2017;17:86-87.
Murakami H, Wang ZI, Marashly A, Krishnan B, Prayson RA, Kakisaka Y, et al. Correlating magnetoencephalography to stereo-electroencephalography in patients undergoing epilepsy surgery. Brain. 2016;139:2935-2947.
Hsiao FJ, Yu HY, Chen WT, Kwan SY, Chen C, Yen DJ, et al. Increased Intrinsic Connectivity of the Default Mode Network in Temporal Lobe Epilepsy: Evidence from Resting-State MEG Recordings. PLoS One. 2015;10:e0128787.
Youssofzadeh V, Agler W, Tenney JR, Kadis DS. Whole-brain MEG connectivity-based analyses reveals critical hubs in childhood absence epilepsy. Epilepsy Res. 2018;145:102-109.
Wu C, Xiang J, Jiang W, Huang S, Gao Y, Tang L, et al. Altered Effective Connectivity Network in Childhood Absence Epilepsy: A Multi-frequency MEG Study. Brain Topogr. 2017;30:673-684.
Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059-1069.
Ali N, Nitschke JP, Cooperman C, Pruessner JC. Suppressing the endocrine and autonomic stress systems does not impact the emotional stress experience after psychosocial stress. Psychoneuroendocrinology. 2017;78:125-130.
Yasuda CL, Chen Z, Beltramini GC, Coan AC, Morita ME, Kubota B, et al. Aberrant topological patterns of brain structural network in temporal lobe epilepsy. Epilepsia. 2015;56:1992-2002.
Lee DA, Kim BJ, Lee HJ, Kim SE, Park KM. Network characteristics of genetic generalized epilepsy: Are the syndromes distinct? Seizure. 2020;82:91-98.
Park KM, Lee BI, Shin KJ, Ha SY, Park J, Kim TH, et al. Progressive topological disorganization of brain network in focal epilepsy. Acta Neurol Scand. 2018;137:425-431.
Sone D, Matsuda H, Ota M, Maikusa N, Kimura Y, Sumida K, et al. Graph Theoretical Analysis of Structural Neuroimaging in Temporal Lobe Epilepsy with and without Psychosis. PLoS One. 2016;11:e0158728.
Doucet GE, Rider R, Taylor N, Skidmore C, Sharan A, Sperling M, et al. Presurgery resting-state local graph-theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia. 2015;56:517-526.
He X, Doucet GE, Pustina D, Sperling MR, Sharan AD, Tracy JI. Presurgical thalamic "hubness" predicts surgical outcome in temporal lobe epilepsy. Neurology. 2017;88:2285-2293.
Liao W, Ji GJ, Xu Q, Wei W, Wang J, Wang Z, et al. Functional Connectome before and following Temporal Lobectomy in Mesial Temporal Lobe Epilepsy. Sci Rep. 2016;6:23153.
Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage. 2009;45(1 Suppl):S199-209.
Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using Support Vector Machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36:1140-1152.
Abbasi B, Goldenholz DM. Machine learning applications in epilepsy. Epilepsia. 2019;60:2037-2047.
Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719-731.
Munsell BC, Wee CY, Keller SS, Weber B, Elger C, da Silva LA, et al. Evaluation of machine learning algorithms for treatment outcome prediction in patients with epilepsy based on structural connectome data. Neuroimage. 2015;118:219-230.
Jin B, Krishnan B, Adler S, Wagstyl K, Hu W, Jones S, et al. Automated detection of focal cortical dysplasia type II with surface-based magnetic resonance imaging postprocessing and machine learning. Epilepsia. 2018;59:982-992.
Kanner AM. Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol. 2016;12:106-116.
Colmers PLW, Maguire J. Network Dysfunction in Comorbid Psychiatric Illnesses and Epilepsy. Epilepsy Curr. 2020;20:205-10.
Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883-890.
Kanner AM. Depression and the risk of neurological disorders. Lancet. 2005;366:1147-1148.
Kessler RC, Angermeyer M, Anthony JC, R DEG, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007:168–176.
Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6:141-146.
Haut SR, Hall CB, Masur J, Lipton RB. Seizure occurrence: precipitants and prediction. Neurology. 2007;69:1905-1910.
Nakken KO, Solaas MH, Kjeldsen MJ, Friis ML, Pellock JM, Corey LA. Which seizure-precipitating factors do patients with epilepsy most frequently report? Epilepsy Behav. 2005;6:85-89.
Privitera M, Walters M, Lee I, Polak E, Fleck A, Schwieterman D, et al. Characteristics of people with self-reported stress-precipitated seizures. Epilepsy Behav. 2014;41:74-77.
Polak EL, Privitera MD, Lipton RB, Haut SR. Behavioral intervention as an add-on therapy in epilepsy: designing a clinical trial. Epilepsy Behav. 2012;25:505-510.
Tang V, Poon WS, Kwan P. Mindfulness-based therapy for drug-resistant epilepsy: An assessor-blinded randomized trial. Neurology. 2015;85:1100-1107.
Ridsdale L, Wojewodka G, Robinson EJ, Noble AJ, Morgan M, Taylor SJC, et al. The effectiveness of a group self-management education course for adults with poorly controlled epilepsy, SMILE (UK): A randomized controlled trial. Epilepsia. 2018;59:1048-1061.
Haut SR, Lipton RB, Cornes S, Dwivedi AK, Wasson R, Cotton S, et al. Behavioral interventions as a treatment for epilepsy: A multicenter randomized controlled trial. Neurology. 2018;90:e963-e970.
Kanner AM. Psychiatric comorbidities in new onset epilepsy: Should they be always investigated? Seizure. 2017;49:79-82.
Bonora A, Benuzzi F, Monti G, Mirandola L, Pugnaghi M, Nichelli P, et al. Recognition of emotions from faces and voices in medial temporal lobe epilepsy. Epilepsy Behav. 2011;20:648-654.
Lv RJ, Sun ZR, Cui T, Guan HZ, Ren HT, Shao XQ. Temporal lobe epilepsy with amygdala enlargement: a subtype of temporal lobe epilepsy. BMC Neurol. 2014;14:194.
Sanchez-Gistau V, Sugranyes G, Bailles E, Carreno M, Donaire A, Bargallo N, et al. Is major depressive disorder specifically associated with mesial temporal sclerosis? Epilepsia. 2012;53:386-392.
Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483-506.
Mohanraj R, Brodie MJ. Outcomes of newly diagnosed idiopathic generalized epilepsy syndromes in a non-pediatric setting. Acta Neurol Scand. 2007;115:204-208.
Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685.
Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62:371–376.
Gloor P. Generalized epilepsy with bilateral synchronous spike and wave discharge. New findings concerning its physiological mechanisms. Electroencephalogr Clin Neurophysiol Suppl. 1978:245–249.
Gomez-Ibanez A, McLachlan RS, Mirsattari SM, Diosy DC, Burneo JG. Prognostic factors in patients with refractory idiopathic generalized epilepsy. Epilepsy Res. 2017;130:69-73.
Szaflarski JP, Lindsell CJ, Zakaria T, Banks C, Privitera MD. Seizure control in patients with idiopathic generalized epilepsies: EEG determinants of medication response. Epilepsy Behav. 2010;17:525-530.
McGill ML, Devinsky O, Kelly C, Milham M, Castellanos FX, Quinn BT, et al. Default mode network abnormalities in idiopathic generalized epilepsy. Epilepsy Behav. 2012;23:353-359.
Zhang Z, Liao W, Chen H, Mantini D, Ding JR, Xu Q, et al. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 2011;134:2912-2928.
Jia X, Ma S, Jiang S, Sun H, Dong D, Chang X, et al. Disrupted Coupling Between the Spontaneous Fluctuation and Functional Connectivity in Idiopathic Generalized Epilepsy. Front Neurol. 2018;9:838.
Liu M, Concha L, Beaulieu C, Gross DW. Distinct white matter abnormalities in different idiopathic generalized epilepsy syndromes. Epilepsia. 2011;52:2267-2275.
Jiang Y, Hu Y, Wang Y, Zhou N, Zhu L, Wang K. Empathy and emotion recognition in patients with idiopathic generalized epilepsy. Epilepsy Behav. 2014;37:139-144.
Chowdhury FA, Elwes RD, Koutroumanidis M, Morris RG, Nashef L, Richardson MP. Impaired cognitive function in idiopathic generalized epilepsy and unaffected family members: an epilepsy endophenotype. Epilepsia. 2014;55:835-840.
Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical journal. 1994;66:259.
Connelly A, Jackson GD, Frackowiak RS, Belliveau JW, Vargha-Khadem F, Gadian DG. Functional mapping of activated human primary cortex with a clinical MR imaging system. Radiology. 1993;188:125-130.
Acknowledgements
This work was supported by the UAB Epilepsy Center. The authors wish to thank Sangeeta Nair, Ayushe Sharma, and Dr. Jane Allendorfer for thoughtful discussions that contributed to the manuscript.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goodman, A.M., Szaflarski, J.P. Recent Advances in Neuroimaging of Epilepsy. Neurotherapeutics 18, 811–826 (2021). https://doi.org/10.1007/s13311-021-01049-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-021-01049-y