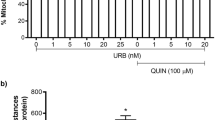
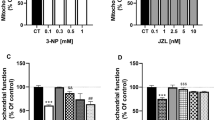
Abstract
Advances in the understanding of the endogenous cannabinoid system have led to several therapeutic indications for new classes of compounds that enhance cannabinergic responses. Endocannabinoid levels are elevated during pathogenic conditions, and inhibitors of endocannabinoid inactivation promote such on-demand responses. The endocannabinoids anandamide and 2-arachidonoyl glycerol have been implicated in protective signaling against excitotoxic episodes, including seizures. To better understand modulatory pathways that can exploit such responses, we used the new generation compound AM6701 that blocks both the anandamide-deactivating enzyme fatty acid amide hydrolase (FAAH) and the 2-arachidonoyl glycerol-deactivating enzyme monoacylglycerol lipase (MAGL) with equal potency. Also studied was the structural isomer AM6702 which is 44-fold more potent for inhibiting FAAH versus MAGL. When applied before and during kainic acid (KA) exposure to cultured hippocampal slices, AM6701 protected against the resulting excitotoxic events of calpain-mediated cytoskeletal damage, loss of presynaptic and postsynaptic proteins, and pyknotic changes in neurons. The equipotent inhibitor was more effective than its close relative AM6702 at protecting against the neurodegenerative cascade assessed in the slice model. In vivo, AM6701 was also the more effective compound for reducing the severity of KA-induced seizures and protecting against behavioral deficits linked to seizure damage. Corresponding with the behavioral improvements, cytoskeletal and synaptic protection was elicited by AM6701, as found in the KA-treated hippocampal slice model. It is proposed that the influence of AM6701 on FAAH and MAGL exerts a synergistic action on the endocannabinoid system, thereby promoting the protective nature of cannabinergic signaling to offset excitotoxic brain injury.









Similar content being viewed by others
References
Hwang J, Adamson C, Butler D, Janero DR, Makriyannis A, Bahr BA. Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: a neuroprotective therapeutic modality. Life Sci 2010;86:615–623.
Zanettini C, Panlilio LV, Alicki M, et al. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci 2011;5:57.
Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 2008;153:252–262.
Janero DR, Vadivel SK, Makriyannis A. Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies. Int Rev Psychiatry 2009;21:122–133.
Chen X, Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neuroscience 2011;178:159–168.
Benito C, Nunez E, Tolon RM, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci 2003;23:11136–11141.
Maccarrone M, Gubellini P, Bari M, et al. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem 2003;85:1018–1025.
Pisani V, Madeo G, Tassone A, et al. Homeostatic changes of the endocannabinoid system in Parkinson's disease. Mov Disord 2011;26:216–222.
Mievis S, Blum D, Ledent C. Worsening of Huntington disease phenotype in CB1 receptor knockout mice. Neurobiol Dis 2011;42:524–529.
Micale V, Mazzola C, Drago F. Endocannabinoids and neurodegenerative diseases. Pharmacol Res 2007;56:382–392.
Ludanyi A, Eross L, Czirjak S, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci 2008;28:2976–2990.
Parmentier-Batteur S, Jin K, Mao XO, et al. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci 2002;22:9771–9775.
Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci 2005;25:7813–7820.
Karanian DA, Karim SL, Wood JT, et al. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J Pharmacol Exp Ther 2007;322:1059–1066.
Hajos N, Katona I, Naiem SS, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci 2000;12:3239–3249.
Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 2001;29:717–727.
Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 2001;29:729–738.
Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 2001;31:453–462.
Deadwyler SA, Hampson RE, Mu J, et al. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther 1995;273:734–743.
Gomez del Pulgar T, Velasco G, Guzman M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J 2000;347:369–373.
Molina-Holgado F, Pinteaux E, Heenan L, et al. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Neurosci 2005;28:189–194.
Galve-Roperh I, Rueda D, Gomez del Pulgar T, et al. Mechanism of extracellular signal-regulated kinase activation by the CB1 cannabinoid receptor. Mol Pharmacol 2002;62:1385–1392.
Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett 2007;14:237–246.
Vandevoorde S, Lambert DM. The multiple pathways of endocannabinoid metabolism: a zoom out. Chem Biodivers 2007;4:1858–1881.
Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol 2008;160:1–24.
Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev 2008;108:1687–1707.
Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and CB1 cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience 2003;119:481–496.
Egertova M, Giang DK, Cravatt BF, et al. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Biol Sci 1998;265:2081–2085.
Gulyas AI, Cravatt BF, Bracey MH, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 2004;20:441–458.
Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA 2002;99:10819–10824.
Hansen HH, Schmid PC, Bittigau P, et al. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem 2001;78:1415–1427.
Marsicano G, Goodenough S, Monory K, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003;302:84–88.
Naidoo V, Nikas SP, Karanian DA, et al. A new generation fatty acid amide hydrolase inhibitor protects against kainate-induced excitotoxicity. J Mol Neurosci 2011;43:493–502.
Hampson RE, Miller F, Palchik G, Deadwyler SA. Cannabinoid receptor activation modifies NMDA receptor mediated release of intracellular calcium: implications for endocannabinoid control of hippocampal neural plasticity. Neuropharmacology 2011;60:944–952.
Onaivi ES. Cannabinoid receptors in brain: pharmacogenetics, neuropharmacology, neurotoxicology, and potential therapeutic applications. Int Rev Neurobiol 2009;88:335–369.
Araujo BH, Torres LB, Cossa AC, et al. Hippocampal expression and distribution of CB1 receptors in the Amazonian rodent Proechimys: an animal model of resistance to epilepsy. Brain Res 2010;1335:35–40.
Alexander JP, Cravatt BF. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem Biol 2005;12:1179–1187.
Vanderklish PW, Bahr BA. The pathogenic activation of calpain: A marker and mediator of cellular toxicity and disease states. Internatl J Exp Pathol 2000;81:323–339.
Bahr BA, Tiriveedhi S, Park GY, Lynch G. Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther 1995;273:902–908.
Ramarao MK, Murphy EA, Shen MW, et al. A fluorescence-based assay for fatty acid amide hydrolase compatible with high-throughput screening. Anal Biochem 2005;343:143–151.
Patricelli MP, Lashuel HA, Giang DK, et al. Comparative characterization of a wild type and transmembrane domain-deleted fatty acid amide hydrolase: identification of the transmembrane domain as a site for oligomerization. Biochemistry 1998;37:15177–15187.
Zvonok N, Pandarinathan L, Williams J, et al. Covalent inhibitors of human monoacylglycerol lipase: ligand-assisted characterization of the catalytic site by mass spectrometry and mutational analysis. Chem Biol 2008;15:854–862.
Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathologic processes. J Neurosci Res 1995;42:294–305.
Araújo IM, Gil JM, Carreira BP, et al. Calpain activation is involved in early caspase-independent neurodegeneration in the hippocampus following status epilepticus. J Neurochem 2008;105:666–676.
Bahr BA, Karanian DA, Makanji SS, Makriyannis A. Targeting the endocannabinoid system in treating brain disorders. Expert Opin Investig Drugs 2006;15:351–365.
Kawahara H, Drew GM, Christie MJ, Vaughan CW. Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey. Br J Pharmacol 2011;163:1214–1222.
Coomber B, O'Donoghue MF, Mason R. Inhibition of endocannabinoid metabolism attenuates enhanced hippocampal neuronal activity induced by kainic acid. Synapse 2008;62:746–755.
Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry 2011;70:479–486.
De Paola V, Arber S, Caroni P. AMPA receptors regulate dynamic equilibrium of presynaptic terminals in mature hippocampal networks. Nat Neurosci 2003;6:491–500.
Gogolla N, Galimberti I, DePaola V, et al. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc 2006;1:1165–1171.
Caba E, Bahr BA. Biphasic NF-κB activation in the excitotoxic hippocampus. Acta Neuropathol 2004;108:173–182.
Ryzhikov S, Bahr BA. Gephyrin alterations due to protein accumulation stress are reduced by the lysosomal modulator Z-Phe-Ala-diazomethylketone. J Mol Neurosci 2008;34:131–139.
Jourdi H, Hamo L, Oka T, Seegan A, Baudry M. BDNF mediates the neuroprotective effects of positive AMPA receptor modulators against MPP + −induced toxicity in cultured hippocampal and mesencephalic slices. Neuropharmacology 2009;56:876–885.
Wu Y, Liang S, Oda Y, et al. Truncations of amphiphysin I by calpain inhibit vesicle endocytosis during neural hyperexcitation. Embo J 2007;26:2981–2990.
David C, McPherson PS, Mundigl O, et al. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA 1996;93:331–335.
Takei K, Slepnev VI, Haucke V, et al. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol 1999;1:33–39.
Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci 2000;1:161–172.
Akaike K, Tanaka S, Tojo H, et al. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res 2001;900:65–71.
Banke TG, Bowie D, Lee H, et al. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 2000;20:89–102.
Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 2000;28:511–525.
Lee HK, Takamiya K, Han JS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 2003;112:631–643.
Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 2006;281:752–758.
Bahr BA, Bendiske J, Brown QB, et al. Survival signaling and selective neuroprotection through glutamatergic transmission. Exp Neurol 2002;174:37–47.
Ortar G, Cascio MG, Moriello AS, et al. Carbamoyl tetrazoles as inhibitors of endocannabinoid inactivation: a critical revisitation. Eur J Med Chem 2008;43:62–72.
Bowman AL, Makriyannis A. Refined homology model of monoacylglycerol lipase: toward a selective inhibitor. J Comput Aided Mol Des 2009;23:799–806.
Long JZ, Nomura DK, Vann RE, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci USA 2009;106:20270–20275.
Alexander SP, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol 2007;152:602–623.
Petzer JP, Castagnoli N, Jr., Schwarzschild MA, et al. Dual-target-directed drugs that block monoamine oxidase B and adenosine A(2A) receptors for Parkinson's disease. Neurotherapeutics 2009;6:141–151.
Pretorius J, Malan SF, Castagnoli N, Jr., et al. Dual inhibition of monoamine oxidase B and antagonism of the adenosine A(2A) receptor by (E,E)-8-(4-phenylbutadien-1-yl)caffeine analogues. Bioorg Med Chem 2008;16:8676–8684.
Venneri A, McGeown WJ, Shanks MF. Empirical evidence of neuroprotection by dual cholinesterase inhibition in Alzheimer's disease. Neuroreport 2005;16:107–110.
Ballard CG. Advances in the treatment of Alzheimer's disease: benefits of dual cholinesterase inhibition. Eur Neurol 2002;47:64–70.
Bartorelli L, Giraldi C, Saccardo M, et al. Effects of switching from an AChE inhibitor to a dual AChE-BuChE inhibitor in patients with Alzheimer's disease. Curr Med Res Opin 2005;21:1809–1818.
Zausinger S, Scholler K, Plesnila N, et al. Combination drug therapy and mild hypothermia after transient focal cerebral ischemia in rats. Stroke 2003;34:2246–2251.
Schmid-Elsaesser R, Hungerhuber E, Zausinger S, et al. Combination drug therapy and mild hypothermia: a promising treatment strategy for reversible, focal cerebral ischemia. Stroke 1999;30:1891–1899.
Acknowledgments
The authors thank Ana Charalambides and Tyler Loehr for their laboratory assistance, and Alyson Bahr for editing services. The work was supported by the National Institutes of Health (grants DA07215, R44 DA023737, and R25 GM077634), the Oliver Smithies Grant from the North Carolina Biotechnology Center (Research Triangle Park, NC), and by funds supporting the William C. Friday Chair. The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Conflict of interest
B.A.B. and A.M. are consultants for a company developing novel fatty acid amide hydrolase (FAAH) inhibitors.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 210 kb)
Rights and permissions
About this article
Cite this article
Naidoo, V., Karanian, D.A., Vadivel, S.K. et al. Equipotent Inhibition of Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase – Dual Targets of the Endocannabinoid System to Protect against Seizure Pathology. Neurotherapeutics 9, 801–813 (2012). https://doi.org/10.1007/s13311-011-0100-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-011-0100-y