Abstract
Introduction
A 60-cm endoscopically implantable duodenal–jejunal bypass liner (Endobarrier™, GI Dynamics, Lexington, MA, USA) has been introduced as a therapeutic option to support weight loss for a selected group of obese subjects with type 2 diabetes mellitus (T2DM). The sleeve prevents contact between chyme and the intestinal mucosa of the upper gastrointestinal tract. The primary aim of this study is to elucidate the changes in insulin sensitivity and beta-cell function after EndoBarrier™ implantation in obese patients with T2DM; changes in gut permeability and gut microbiome are also to be examined.
Methods
This is an open, single-center, prospective trial in which ten obese subjects with T2DM and suboptimal glycemic control (glycosylated hemoglobin A1c (HbA1c) level > 48 mmol/mol) are investigated with regards to EndoBarrier™ implantation. The Endobarrier™ is implanted shortly after baseline and left in situ for a period of 36 weeks. Dual-energy X-ray absorptiometry measurement, assessment of beta-cell function and insulin sensitivity as measured by a Botnia clamp procedure, and a mixed-meal tolerance test are performed prior to implantation and at 4, 36, and 64 weeks after implantation. The composition of the gut microbiota is characterized from stool using 454 pyrosequencing of 16S rRNA genes. Gut permeability is assessed by a differential sugar absorption method.
Planned outcome
This study will give mechanistic insights in particulr into changes of insulin sensitivity, beta-cell function or microbiome changes over time in subjects implanted with an EndobarrierTM device.
Trial registration
NCT02769728, Registered 12 May 2016. Current Protocol Date/Version: 04 September 2017/Version 1.9.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has reached epidemic proportions in the Western world and has risen to the top of the public health policy agendas of many countries. The World Health Organization (WHO) predicts that approximately 2.7 billion adults will be overweight (body mass index [BMI] 25–29.9 kg/m2) by 2025, and worldwide more than 177 million adults will be severely obese and in need of treatment (BMI ≥ 35 kg/m2) [1].
The association between obesity and type 2 diabetes mellitus (T2DM) is well recognized. A higher BMI is a strong predictor for T2DM [2].
The current estimate is that 285 million people worldwide suffer from T2DM, and this number is anticipated to increase to 439 million by 2030 [3]. T2DM raises the risk for cardiovascular events, heart failure, eye problems, and chronic kidney disease. Previous research has demonstrated that the impact of diabetes on everyday life and the likelihood of costly and disabling complications can be minimized by more intensive management of glucose level [4], blood pressure [5], and cholesterol level [6].
Bariatric surgery (BS) was established as a treatment option for morbidly obese patients or obese subjects with comorbidities such as T2DM. In addition to reducing body weight, BS has been reported to improve glycemic control in patients with T2DM and, most importantly, to reduce cardiovascular events and overall mortality in obese subjects [7,8,9,10]. Glycemic improvement has been reported to occur rapidly within the first weeks after BS, an effect which cannot be explained by the relatively small weight loss seen during this short time period. These beneficial glycemic changes after bypass procedures have been attributed to changes in incretin levels and the interplay of other gut hormones [11].
In 2010 GI Dynamics (Lexington, MA, USA) introduced a duodenal–jejunal bypass liner (DJBL), the Endobarrier™, which had received its certification mark (CE mark). The EndoBarrier™ is a 60-cm-long impermeable fluoropolymer sleeve-like device that is placed endoscopically via the oral route and anchored in the duodenum, creating a duodenal–jejunal bypass, for a maximum of 12 months. It allows the transit of chyme from the stomach through the jejunum without any contact to the duodenal mucosa. Both bile and pancreatic exocrine secretions do not come into contact with chyme in the proximal jejunum; consequently, the DJBL mimics a duodenal–jejunal bypass and therefore induces weight loss.
A number of studies have demonstrated the feasibility of implanting this device, with the patient experiencing subsequent weight reduction [12,13,14,15,16]. Both significant improvements in glycemic parameters and improvements in insulin resistance, as measured by the homeostatic model assessment-insulin resistance (HOMA-IR), have been observed in T2DM patients after EndoBarrier™ implantation [17, 18].
However, a thorough investigation of insulin resistance and beta-cell function in patients with the Endobarrier™ device using the gold standard, namely, an intravenous glucose tolerance test combined with a hyperinsulinemic–euglycemic clamp (Botnia clamp), is lacking to date. The aim of the study described here is to explore the short- and longer-term effects (26 weeks after removal) of the EndoBarrier™ implantation on insulin resistance and beta-cell function as assessed by repeated Botnia clamps to further delineate the mechanisms of this intervention.
The ENDO trial investigating the effects of the Endobarrier™ device compared to a sham intervention was terminated early due to a higher rate of hepatic abscesses than expected, leading to speculations on the factors leading to this adverse effect. In this context, we discuss a leaky gut and changes in the upper gastrointestinal microbiome caused by the bypass-liner. Therefore, a second aim of this study is to investigate potential changes in microbiome composition and gut permeability.
Methods
Study Design
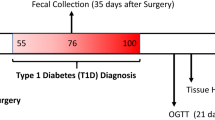
This is an open, single-center, prospective, single-arm pilot study aimed at evaluating the effect of EndoBarrier™ implantation on insulin sensitivity and beta-cell function in obese patients with T2DM over a period of 36 weeks (Fig. 1). The investigations are performed at the Medical University of Graz (academic hospital), Department of Internal Medicine, Division of Endocrinology and Diabetology as well as at the Division of Gastroenterology and Hepatology.
Inclusion and Exclusion Criteria
The study population consists of ten subjects with T2DM, based on American Diabetes Association (ADA) criteria [19] and a BMI of between 30.0 and 49.0 kg/m2. Participants are aged 18–70 years and have a HbA1c level of > 6.5% (48 mmol/mol). Patients with all options of antidiabetic therapy (diet, oral antidiabetic drugs, insulin) were eligible for the trial, with the exception of users of glucagon-like peptide-1 (GLP-1) receptor agonist therapy. The main exclusion criteria were: type 1 diabetes; pregnancy or intention to become pregnant; use of non-steroidal anti-inflammatory drugs, anticoagulation therapy, or corticosteroids; use of weight loss medication; use of drugs affecting gastrointestinal motility; or previous gastrointestinal tract surgery. Obese patients were identified from their attendance at the obesity outpatient clinic at the Department of Endocrinology and Diabetology.
Primary Objective
The primary objective of this trial is to determine the changes in insulin sensitivity and beta-cell function after EndoBarrier™ implantation during a follow-up of 36 weeks in patients with T2DM and a BMI of between 30.0 and 49.0 kg/m2.
Secondary Objectives
-
1.
To assess the beta-cell insulin response to oral and intravenous glucose loads early (4 weeks) and at 36 weeks after EndoBarrier™ implantation as well as 26 weeks after removal of the duodenal–jejunal bypass device.
-
2.
To assess changes in body weight from baseline to 4, 36, and 62 weeks, respectively, after implantation of the duodenal–jejunal bypass device.
-
3.
To determine changes in gut permeability and gut microbiome composition from baseline to 4, 36, and 62 weeks, respectively, after implantation of the duodenal–jejunal bypass device (DJBL).
-
4.
To evaluate changes in the area under the curve (AUC) of glucose from baseline to 4, 36, and 62 weeks, respectively, after implantation of the duodenal–jejunal bypass device.
-
5.
To assess changes in body composition as assessed by dual-energy X-ray absorptiometry (DEXA) from baseline to 4, 36, and 62 weeks, respectively, after implantation of the duodenal–jejunal bypass device.
-
6.
To evaluate correlations between mixed meal tolerance test (MMTT) and Botnia clamp results regarding insulin sensitivity and beta-cell function.
-
7.
To determine changes in cardiovascular risk as assessed by the United Kingdom Prospective Diabetes Study (UKPDS) risk engine from baseline to 4, 36, and 62 weeks, respectively, after implantation of the duodenal–jejunal bypass device.
Intervention
The EndoBarrier™ or DJBL consists of a nickel–titanium anchor and a 60-cm-long impermeable sleeve made of fluoropolymer. It is endoscopically placed in the duodenum through an over-the-wire system and then fixed to the intestinal wall within the duodenal bulb, thus proximal to the intestinal orifice of the bile duct. Chyme passes through the EndoBarrier™, but the bile and pancreatic enzymes pass outside of the liner and will mix with the chyme at the end of the EndoBarrier™. During the implantation process of the EndoBarrier™ device, eight gastric and small bowel biopsies are taken. Four biopsies are used for histology studies, and the other four biopsies are used for RNA extraction to perform genome-wide expression analysis. Implantation and removal of the EndoBarrier™ device requires fluoroscopic X-ray guidance to determine the position of the device.
Visit Schedule
A summary of all study visits and procedures is shown in Table 1.
Diet and Supplementation
Subjects are instructed to start taking a proton pump inhibitor (omeprazole 40 mg twice-daily) orally 3 days prior to the implantation procedure of the Endobarrier™ device and asked to continue with the medication until 2 weeks after the removal of the device. Additionally, all patients receive a daily multivitamin preparation (Supradyn®; Bayer, Leverkusen, Germany). At hospital discharge after the implantation process, subjects are instructed to follow a liquid diet during the first 2 weeks following implantation and to gradually transit to a normal diet over the next 10 days. The instructions are given by a certified nutritionist.
Mixed Meal Tolerance Test
The MMTT is performed after an overnight fast (apart from water). A pre-meal blood sample is taken (− 5 min), and then all subjects are asked to drink Fortimel® Compact (10 kcal/kg; Nutricia, Danone, Paris, France) over a period of 2–4 min (beginning at time 0 min). During the meal test further blood samples are taken at four time points (15, 30, 60, and 120 min after drinking Fortimel Compact®). Glucose, Insulin, and C-peptide levels are determined in all samples.
Blood Sampling
Routine parameters are determined using a Cobas analyzer (Roche Diagnostics, Mannheim, Germany). Plasma levels of adiponectin are measured by an enzyme-linked immunosorbent assay (ELISA), according to the procedures supplied by the manufacturer (BioVendor, BrnoŘečkovice, Czech Republic).
Botnia Clamp (Combined Intravenous Glucose Tolerance Test and Hyperinsulinemic–Euglycemic Clamp)
The Botnia clamp is a validated design for assessment of beta-cell function and insulin sensitivity [20]. After an overnight fast of at least 8 h and after baseline samples are obtained, subjects are given a 20% glucose solution (0.3 g/kg body weight) at time 0 min. Blood samples for the measurement of plasma glucose, insulin, and C-peptide are obtained at − 10, 0, 2, 4, 6, 8, 10, 20, 30, 40, 50, and 60 min. At 60 min after administration of the glucose bolus, hyperinsulinemic–euglycemic clamp is started to evaluate insulin sensitivity. A priming dose of insulin (3 IU/m2) followed by an infusion (40 mU/m2/min) of short-acting human insulin is applied for 120 min. Blood glucose is clamped at the concentration of 5.0 ± 0.5 mmol/l by a variable infusion of 20% glucose. Blood samples for measurement of plasma glucose concentrations and for measurement of insulin concentrations are obtained at 5- and 30-min intervals, respectively, throughout the clamp. The mean amount of glucose infused during the last 60 min of the euglycemic clamp is used to calculate the rate of whole-body glucose uptake. Glucose concentration in arterialized blood samples is measured in duplicate using the Hitado Super GL compact analyzer (Hitado, Möhnesee, Germany). Insulin and C-peptide levels are measured using routinely available chemiluminescence on an ADVIA Centaur system (Siemens Healthcare Diagnostics, Eschborn, Germany).
GLP-1 Analysis
Blood samples are to be collected into pre-chilled tubes containing EDTA + aprotoinin. After centrifugation, plasma samples are frozen at − 80 °C until analysis. For the determination of human glucagon like peptide 1 (GLP-1) a commercially available ELISA kit is used (active GLP-1 ELISA (GLP-1 (7-36) and (9-36), ALPCO Diagnostics, Salem, NH, USA). The test is performed according the instructions provided by the distributor.
Dual-Energy X-Ray Absorptiometry
Dual-energy X-ray absorptiometry measurement is performed with a full-size GE Lunar iDEXA densitometer (GE Healthcare, Waukesha, WI, USA) for the purpose of estimating the percentage body fat. The measurements are carried out in accordance to the departmental Standard Operating Procedure. Body regions are defined using standard anatomical partitions. Scan areas are analyzed to determine lean mass, fat mass, bone mineral content, and total body fluid percentage.
Determination of Gut Permeability
After overnight fasting, patients are encouraged to drink 100 ml of a solution containing 10 g lactulose, 5 g mannitol, and 20 g saccharose in the morning. Urine is collected over a 5-h period thereafter while fasting is continued. Patients are not allowed to drink within the first 2 h after ingestion of the sugar solution. The urine volume collected within 5 h is measured, and 1-ml aliquots are frozen at − 80 °C after the addition of 100 ml of thimerosal (10 mg/ml; Sigma-Aldrich Handels GmbH, Vienna, Austria) for subsequent analysis.
DNA Isolation, 454 Library Preparation, and Sequencing
Total DNA is isolated from frozen stool samples using the MagnaPure LC DNA Isolation Kit III (Bacteria, Fungi) (Roche Diagnostics) according to the manufacturer’s instructions, including mechanic and enzymatic lysis as described in Klymiuk et al. [21]. For 16S rRNA gene analysis, hypervariable regions V1–2 are amplified in a target-specific PCR assay (primers: 27F-AGAGTTTGATCCTGGCTCAG; R357-CTGCTGCCTYCCGTA), and amplification products are sequenced after indexing and purification on an Illumina MiSeq desktop sequencer (Illumina, Eindhoven, The Netherlands) according to published procedures [21, 22] at the Core Facility for Molecular Biology at the Center for Medical Research in Graz.
Food Frequency Questionnaire
A self-administered, semi-quantitative food frequency questionnaire was developed to assess usual food consumption within the German Health Examination Survey for Adults 2008–2011 (DEGS) [23]. The relative validity of this questionnaire has been studied among participants of another nationwide survey, the German National Nutrition Monitoring (NEMONIT) [24].
Statistical Assessment
The combination of MMTT and intravenous glucose tolerance test (IVGTT) provides a comprehensive assessment of different aspects of beta-cell function as well as a reliable measurement of insulin sensitivity assessed during the euglycemic clamp procedure [25]. The following empirical indices of beta-cell function will be derived from the MMTT data: (1) insulinogenic index (IGI), calculated as (30 min insulin − basal insulin)/(30 min glucose − basal glucose); (2) the early insulin response, calculated by using the formula (30 min insulin − basal insulin)/(30 min glucose) [26]; or (3) ratio of AUC for C-peptide divided by AUC for glucose (AUCCP/AUCGLU). AUCs are determined by trapezoidal method. Based on IVGTT measurements, the incremental trapezoidal area of insulin and C-peptide during the first 10 min after glucose administration will represent the first-phase insulin response or acute insulin response (AIR). AIR will be also measured as the sum of insulin and C-peptide concentrations during the first 10 min after the glucose challenge. The incremental insulin/C-peptide secretion during the last 50 min of IVGTT will represent the second-phase insulin secretion. AIR and rate sensitivity as well as insulin secretion rates will be used to evaluate beta-cell insulin response to oral versus intravenous glucose load. Insulin sensitivity will be assessed as the mean glucose uptake, calculated from the glucose infusion rates during the last 60 min of the euglycemic clamp (M-value) and as the ratio of glucose infusion rates during the last 60 min of the clamp and the mean steady state insulin levels during the same interval (M/I = SI Index).
Changes in body weight will be analyzed as the difference in absolute total body weight, change in BMI, excess weight loss, and total body weight loss. Changes in T2DM will be analyzed as the absolute change in HbA1c, fasting plasma glucose, and changes in T2DM medication. Differences in lipid levels and blood pressure will be analyzed as absolute differences.
All data will be checked for distribution normality. Changes in beta-cell function and other variables after EndoBarrier™ implantation will be assessed by analysis of variance with repeated measures.
Analyses will be conducted with IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Missing values will not be imputed. Data will be reported as mean values with standard deviation within parentheses, unless specified otherwise. A p value of < 0.05 will be considered to be statistically significant. Analyses between different time points will be conducted with a paired-sample t test, or with the Wilcoxon signed rank test when data are not normally distributed.
Sample Size
This study is planned as a mechanistic pilot trial with the aim to gather preliminary data on the parameters outlined in the preceding sections. Currently there is insufficient data available which would allow calculation of the sample size needed. However, previous pilot trials investigating measurements of glucose metabolism with EndoBarrier™ have used 10–16 subjects to demonstrate improvements [27, 28]. Therefore, our aim is to include ten subjects.
Strengths and Limitations
The present trial is the first to investigate the impact of Endobarrier™-implantation on insulin resistance and beta-cell function with the gold standard, namely an intravenous glucose tolerance test combined with a hyperinsulinemic–euglycemic clamp (Botnia clamp). Additionally, our interventional phase was reduced to 9 months of Endobarrier™ in situ after the risk for increased incidence for liver abscesses became evident. A major limitation is that we perform a pilot study which will serve as a basis for further larger studies.
Ethics and Dissemination
The study is to be performed according to the principles of the International Declaration of Helsinki and to the principles of good clinical practice and has been approved by the ethics committee of the Medical University of Graz (26-280 ex 13/14). The trial is conducted at the Division of Endocrinology and Diabetology at the Medical University of Graz (academic hospital), Austria. The protocol is registered in ClinicalTrials.gov with the identifier NCT02769728. Clinical trial authorization was obtained from the Austrian Agency for Health and food Safety (AGES; INS-621000-0620). Important protocol modifications are reported to the local Ethics Committee of the Medical University of Graz and if necessary to the AGES.
Participants are asked to sign informed consent before being enrolled into the trial, in compliance with the Declaration of Helsinki and WHO standards. Subjects are informed of the study objectives and the risks and benefits of the examinations they will undergo. All subjects are given sufficient time to decide whether they wish to participate in the trial.
The trial includes collection of biological samples (blood, urine, stool, sputum), of which subjects are informed in detail. The samples are stored at − 80 °C for use in future research at the Biobank of the Medical University of Graz.
Dissemination Plan
Our aim is to disseminate the results of this study through peer-reviewed journals and national and international academic conferences.
Discussion
The results of this study will provide insights into the mechanisms of glucose metabolism after DJBL implantation. Insulin sensitivity and declining beta-cell function are the key pathophysiologic drivers in T2DM. Previous reports have demonstrated improvements in both parameters in subjects undergoing BS [29], but as yet the effects of a gastro-duodenal bypass liner on these key determinants of glucose metabolism are not well investigated. The results of the first of such studies suggest that positive effects on glucose metabolism occur rapidly after DJBL implantation [27] and that these effects are comparable to those after BS. However, these studies used MMTTs to estimate insulin resistance, and this type of oral test may not be the appropriate assessment tool in an intervention which prevents direct contact between chyme and the intestinal wall of the upper gastrointestinal tract. Our study will add to the understanding of glucose metabolism by thoroughly assessing beta-cell function and insulin sensitivity by mean of a Botnia clamp procedure in addition to a MMTT.
In addition, we expect to gain first insights into changes in gut permeability and gut microbiome composition during this intervention, which will facilitate our understanding of the mechanisms of action of DJBL. Additionally, we will also be able to gain more insight into safety aspects of DJBL, which may be involved in the pathogenesis of the moderately increased risk for hepatic abscess associated with the gastro-duodenal sleeve implantation [30]. A recent review that included 1056 patients graded the majority of adverse events (AEs) associated with gastro-duodenal sleeve implantation as mild, including nausea and vomiting, with 20.7% of AEs classified as moderate (e.g., migration, ulceration); 33 events were classified as severe AEs, with hepatic abscess, gastrointestinal hemorrhage, and esophageal perforation being the most frequent events [31]. Most of the reported AEs consist of mild general gastrointestinal complaints that occurred during the first 2 weeks after endoscopic implantation [31]. As hepatic abscesses occurred later on during the Endobarrier™ treatment in the ENDO trial, we will limit the therapy duration to 9 months for safety reasons.
References
Coutinho WL. World obesity day press release. London: World Obesity Federation; 2015.
Stern MP, Haffner SM. Body fat distribution and hyperinsulinemia as risk factors for diabetes and cardiovascular disease. Arteriosclerosis. 1986;6:123–30.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13.
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96.
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85.
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76.
Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65.
Laferrere B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes. 2011;35(Suppl 3):S22–5.
Gersin KS, Rothstein RI, Rosenthal RJ, Stefanidis D, Deal SE, Kuwada TS, et al. Open-label, sham-controlled trial of an endoscopic duodenojejunal bypass liner for preoperative weight loss in bariatric surgery candidates. Gastrointest Endosc. 2010;71:976–82.
Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, et al. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251:236–43.
Escalona A, Pimentel F, Sharp A, Becerra P, Slako M, Turiel D, et al. Weight loss and metabolic improvement in morbidly obese subjects implanted for 1 year with an endoscopic duodenal-jejunal bypass liner. Ann Surg. 2012;255(6):1080–5.
Betzel B, Homan J, Aarts EO, Janssen IMC, de Boer H, Wahab PJ, et al. Weight reduction and improvement in diabetes by the duodenal-jejunal bypass liner: a 198 patient cohort study. Surg Endosc. 2017;31:2881–91.
Koehestanie P, Dogan K, Berends F, Janssen I, Wahab P, Groenen M, et al. Duodenal-jejunal bypass liner implantation provokes rapid weight loss and improved glycemic control, accompanied by elevated fasting ghrelin levels. Endosc Int Open. 2014;2:E21–7.
de Moura EG, Martins BC, Lopes GS, Orso IR, de Oliveira SL, Galvao Neto MP, et al. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal-jejunal bypass liner. Diabetes Technol Ther. 2012;14:183–9.
Jirapinyo P, Haas AV, Thompson CC. Effect of the duodenal-jejunal bypass liner on glycemic control in patients with type 2 diabetes with obesity: a meta-analysis with secondary analysis on weight loss and hormonal changes. Diabetes Care. 2018;41:1106–15.
American Diabetes Association (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38[Suppl]:S8–16.
Tripathy D, Wessman Y, Gullstrom M, Tuomi T, Groop L. Importance of obtaining independent measures of insulin secretion and insulin sensitivity during the same test: results with the Botnia clamp. Diabetes Care. 2003;26:1395–401.
Klymiuk I, Bilgilier C, Stadlmann A, Thannesberger J, Kastner MT, Hogenauer C, et al. The human gastric microbiome is predicated upon infection with Helicobacter pylori. Frontiers in microbiology. 2017;8:2508.
Klymiuk I, Bambach I, Patra V, Trajanoski S, Wolf P. 16S based microbiome analysis from healthy subjects’ skin swabs stored for different storage periods reveal phylum to genus level changes. Front Microbiol. 2016;7:2012.
Robert-Koch-Institut. DEGS—Studie zur Gesundheit Erwachsener in Deutschland. Berlin: Robert Koch Institut; 2009.
Haftenberger M, Heuer T, Heidemann C, Kube F, Krems C, Mensink GB. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr J. 2010;9:36.
Ferrannini E, Mari A. Beta cell function and its relation to insulin action in humans: a critical appraisal. Diabetologia. 2004;47(5):943–56.
Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med. 1995;12(10):931.
de Jonge C, Rensen SS, Verdam FJ, Vincent RP, Bloom SR, Buurman WA, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg. 2013;23:1354–60.
Rodriguez L, Reyes E, Fagalde P, Oltra MS, Saba J, Aylwin CG, et al. Pilot clinical study of an endoscopic, removable duodenal-jejunal bypass liner for the treatment of type 2 diabetes. Diabetes Technol Ther. 2009;11:725–32.
Camastra S, Vitali A, Anselmino M, Gastaldelli A, Bellini R, Berta R, et al. Muscle and adipose tissue morphology, insulin sensitivity and beta-cell function in diabetic and nondiabetic obese patients: effects of bariatric surgery. Sci Rep. 2017;7:9007.
Maggi U, Formiga A, Lauro R. Hepatic abscess as a complication of duodenal-jejunal bypass sleeve system and review of the literature. Surg Obes Relat Dis. 2016;12:e47–50.
Betzel B, Drenth JPH, Siersema PD. Adverse events of the duodenal-jejunal bypass liner: a systematic review. Obes Surg. 2018;28:3669–77.
Acknowledgements
We are very grateful to our study participants for their participation.
Funding
The study was funded by the Joseph-Skoda Award of the Austrian Society of Internal Medicine to cover the costs for staff and laboratory analyses. Training for Endobarrier™ device implantation and supervision at implantations were provided by GI Dynamics, Boston, MA, USA. The article processing charges were funded by the research group of HS.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
HS, NJT, and TRP designed and wrote the study protocol and obtained authorization from the Ethics Committee. HS, JU, PP, AMO, CS, and HK perform out-patient visits and edited the manuscript. FS, CH, and AE perform implantations and removals of the DJBL and edited the manuscript. FA recruited patients, performs out-patient visits, and edited the manuscript. VS works on the gut permeability and microbiome analyses and edited the manuscript. ES and MB perform the clamp examinations and laboratory analysis and edited the manuscript. All authors made substantial contributions to the conception and design of this manuscript.
Disclosures
All authors (Norbert Joachim Tripolt, Felix Aberer, Jasmin Url, Christoph Högenauer, Florian Schreiber, Andreas Eherer, Caren Sourij, Anna-Maria Obermayer, Vanessa Stadlbauer, Eva Svehlikova, Martina Brunner, Harald Kojzar, Peter Nikolaus Pferschy, Thomas Rudolf Pieber, and Harald Sourij) have nothing to disclose related to this manuscript.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to: https://doi.org/10.6084/m9.figshare.7327145.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tripolt, N.J., Aberer, F., Url, J. et al. Impact of Duodeno-Jejunal Bypass Liner (EndoBarrierTM) Implantation on Insulin Sensitivity in Patients with Type 2 Diabetes Mellitus (T2DM): A Study Protocol for a Pilot Trial. Diabetes Ther 10, 299–309 (2019). https://doi.org/10.1007/s13300-018-0540-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-018-0540-z