Abstract
Purpose
Imeglimin is the first in a new class of oral antidiabetic agents, the glimins, currently in development to improve glycemic control in patients with type 2 diabetes mellitus. A thorough QT study was conducted to establish electrophysiological effects of therapeutic and supratherapeutic doses of imeglimin on cardiac repolarization.
Methods
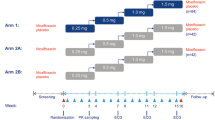
In this randomized, double-blind, four-period, placebo and active controlled crossover study, healthy subjects were administered a single dose of imeglimin 2250 mg, imeglimin 6000 mg, moxifloxacin 400 mg, and placebo. 12-Lead Holter ECGs were recorded from 1 h before dosing until at least 24 h after each dose. This study was performed at a single-center inpatient clinical pharmacology unit.
Results
The upper bound of the two-sided 90% confidence interval for time-matched, placebo-subtracted, baseline-adjusted QTc intervals (ΔΔQTcF) did not exceed the regulatory threshold of 10 ms in any of the imeglimin dose groups. There were no QTcF values above 500 ms nor changes from pre-dose in QTcF above 60 ms in the imeglimin groups. Imeglimin did not exert a relevant effect on heart rate and PR or QRS intervals. Assay sensitivity was demonstrated by the effect of moxifloxacin 400 mg, with a lower bound two-sided 90% confidence interval for ΔΔQTcF of 10.6 ms.
Conclusion
This thorough QT study demonstrated that therapeutic and supratherapeutic exposures of imeglimin did not induce a QT/QTc prolongation with a strong confidence as evidenced by the assay sensitivity.
Trial registration number/date
NCT02924337/ October 5, 2016



Similar content being viewed by others
References
Matthaei S, Stumvoll M, Kellerer M, Haring HU (2000) Pathophysiology and pharmacological treatment of insulin resistance. Endocrine reviews 21(6):585–618. https://doi.org/10.1210/edrv.21.6.0413
Meier JJ, Butler PC (2005) Insulin secretion. In: Endocrinologyed. Elservier Saunders.
(1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England) 352(9131):837–853
WHO (2016) Global report on Diabetes
The Japan Diabetes Society (2013) Evidence-based practice guidelines for diabetes in Japan
Pirags V, Lebovitz H, Fouqueray P (2012) Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab 14(9):852–858. https://doi.org/10.1111/j.1463-1326.2012.01611.x
Vial G, Chauvin MA, Bendridi N, Durand A, Meugnier E, Madec AM, Bernoud-Hubac N, Pais de Barros JP, Fontaine É, Acquaviva C, Hallakou-Bozec S, Bolze S, Vidal H, Rieusset J (2015) Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes 64(6):2254–2264. https://doi.org/10.2337/db14-1220
Pacini G, Mari A, Fouqueray P, Bolze S, Roden M (2015) Imeglimin increases glucose-dependent insulin secretion and improves β-cell function in patients with type 2 diabetes. Diabetes Obes Metab 17(6):541–545. https://doi.org/10.1111/dom.12452
Detaille D, Vial G, Borel AL, Cottet-Rouselle C, Hallakou-Bozec S, Bolze S, Fouqueray P, Fontaine E (2016) Imeglimin prevents human endothelial cell death by inhibiting mitochondrial permeability transition without inhibiting mitochondrial respiration. Cell death discovery 2:15072. https://doi.org/10.1038/cddiscovery.2015.72
Bolze S (2017) Safety, tolerability and pharmacokinetics of Imeglimin in healthy Japanese subjects. Asian Association for the Study of Diabetes (AASD), Nagoya, 19-20 May
Fouqueray P (2015) Dose ranging-study to determine the optimum dose for imeglimin, a novel treatment for type 2 diabetes.American Diabetes Association (ADA), Boston, 5-9 June
Dubourg J (2017) Imeglimin monotherapy in Japanese patients with type 2 diabetes: results from a randomised, 24-week, double-blind, placebo-controlled, phase IIb trial. European Association for the Study of Diabetes (EASD), Lisbon, 11-15 September
Dubourg J (2019) Clinical evidence to support the safety and efficacy of imeglimin in various population of patients with type 2 diabetes. European Association for the Study of Diabetes (EASD), Barcelona, 16-20 September
ICH (2005) International conference on harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Federal register 70(202):61134–61135
(1997) World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Jama 277(11):925–926
Funding
This study was sponsored by Poxel. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Author information
Authors and Affiliations
Contributions
JD contributed to the interpretation of data and drafted and edited the report. SP, MF, SB, PV, and PF contributed to the study design and interpretation of data and reviewed the report. All authors read the manuscript critically and approved the submitted version.
Corresponding author
Ethics declarations
This clinical trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines [15] and was approved by an Independent Ethics Committee/Institutional Review Board (Office for Research Ethics Committees Northern Ireland - ORECNI). All participants provided written informed consent prior to any trial-related activities.
Conflict of interest
JD, SP, SB, and PF are employees of Poxel. MF is an employee of Phinc development. PV is an employee of Banook Group.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dubourg, J., Perrimond-Dauchy, S., Felices, M. et al. Absence of QTc prolongation in a thorough QT study with imeglimin, a first in class oral agent for type 2 diabetes mellitus. Eur J Clin Pharmacol 76, 1393–1400 (2020). https://doi.org/10.1007/s00228-020-02929-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-02929-6