Abstract
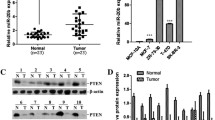
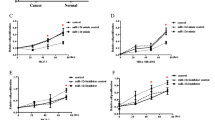
MicroRNAs (miRNAs) have emerged as important regulators that potentially play critical roles in cancer cell biological processes. Previous studies have shown that miR-492 plays an important role in cell tumorigenesis in multiple kinds of human cancer cells. However, the underlying mechanisms of this microRNA in breast cancer remain largely unknown. In the present study, we investigated miR-492’s role in cell proliferation of breast cancer. MiR-492 expression was markedly upregulated in breast cancer tissues and breast cancer cells. Overexpression of miR-492 promoted the proliferation and anchorage-independent growth of breast cancer cells. Bioinformatics analysis further revealed sex-determining region Y-box 7 (SOX7), a putative tumor suppressor, as a potential target of miR-492. Data from luciferase reporter assays showed that miR-492 directly binds to the 3′-untranslated region (3′-UTR) of SOX7 messenger RNA (mRNA) and repressed expression at both transcriptional and translational levels. Ectopic expression of miR-492 led to downregulation of SOX7 protein, which resulted in the upregulation of cyclin D1 and c-Myc. In functional assays, SOX7 silenced in miR-492-in-transfected ZR-75-30 cells has positive effect to promote cell proliferation, suggesting that direct SOX7 downregulation is required for miR-492-induced cell proliferation and cell cycle of breast cancer. In sum, these results suggest that miR-492 represents a potential onco-miR and participates in breast cancer carcinogenesis by suppressing SOX7 expression.





Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Dalmay T. Mechanism of miRNA-mediated repression of mRNA translation. Essays Biochem. 2013;54:29–38.
van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–56.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66.
Li P, Xie XB, Chen Q, Pang GL, Luo W, Tu JC, et al. MiRNA-15a mediates cell cycle arrest and potentiates apoptosis in breast cancer cells by targeting synuclein-gamma. Asian Pac J Cancer Prev: APJCP. 2014;15:6949–54.
Krutilina R, Sun W, Sethuraman A, Brown M, Seagroves TN, Pfeffer LM, et al. MicroRNA-18a inhibits hypoxia-inducible factor 1-alpha activity and lung metastasis in basal breast cancers. Breast Cancer Res. 2014;16:R78.
Paulmurugan R. MicroRNAs—a new generation molecular targets for treating cellular diseases. Theranostics. 2013;3:927–9.
Zhang W, Liu J, Wang G. The role of microRNAs in human breast cancer progression. Tumour Biol J Int Soc Oncodev Biol Med. 2014;35:6235–44.
von Frowein J, Pagel P, Kappler R, von Schweinitz D, Roscher A. MicroRNA-492 is processed from the keratin 19 gene and up-regulated in metastatic hepatoblastoma. Hepatology (Baltimore, Md). 2011;53:833–42.
Zhao JJ, Yang J, Lin J, Yao N, Zhu Y, Zheng J, et al. Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Childs Nerv Syst: ChNS: Off J Int Soc Pediatr Neurosurg. 2009;25:13–20.
Hui AB, Lin A, Xu W, Waldron L, Perez-Ordonez B, Weinreb I, et al. Potentially prognostic miRNAs in HPV-associated oropharyngeal carcinoma. Clin Cancer Res: Off J Am Assoc Cancer Res. 2013;19:2154–62.
Wu GG, Li WH, He WG, Jiang N, Zhang GX, Chen W, et al. MiR-184 post-transcriptionally regulates SOX7 expression and promotes cell proliferation in human hepatocellular carcinoma. PLoS One. 2014;9:e88796.
Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of SOX7 is associated with aberrant activation of Wnt/β-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–56.
Zhang Y, Huang S, Dong W, Li L, Feng Y, Pan L, et al. SOX7, down-regulated in colorectal cancer, induces apoptosis and inhibits proliferation of colorectal cancer cells. Cancer Lett. 2009;277:29–37.
Rajabi HN, Takahashi C, Ewen ME. Retinoblastoma protein and MyoD function together to effect the repression of fra-1 and in turn cyclin D1 during terminal cell cycle arrest associated with myogenesis. J Biol Chem. 2014;289:23417–27.
Cui J, Xi H, Cai A, Bian S, Wei B, Chen L. Decreased expression of SOX7 correlates with the upregulation of the Wnt/beta-catenin signaling pathway and the poor survival of gastric cancer patients. Int J Mol Med. 2014;34:197–204.
Stovall DB, Wan M, Miller LD, Cao P, Maglic D, Zhang Q, et al. The regulation of SOX7 and its tumor suppressive role in breast cancer. Am J Pathol. 2013;183:1645–53.
Stovall DB, Cao P, Sui G. SOX7: from a developmental regulator to an emerging tumor suppressor. Histol Histopathol. 2014;29:439–45.
He M, Li Y, Zhang L, Li L, Shen Y, Lin L, et al. Curcumin suppresses cell proliferation through inhibition of the Wnt/beta-catenin signaling pathway in medulloblastoma. Oncol Rep. 2014;32:173–80.
Zhi X, Tao J, Xie K, Zhu Y, Li Z, Tang J, et al. Muc4-induced nuclear translocation of beta-catenin: a novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett. 2014;346:104–13.
Lee MA, Park HJ, Chung HJ, Kim WK, Lee SK. Antitumor activity of 2-hydroxycinnamaldehyde for human colon cancer cells through suppression of beta-catenin signaling. J Nat Prod. 2013;76:1278–84.
Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, et al. c-Myc associates with ribosomal DNA and activates RNA polymerase i transcription. Nat Cell Biol. 2005;7:303–10.
Grewal SS, Li L, Orian A, Eisenman RN, Edgar BA. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat Cell Biol. 2005;7:295–302.
Acknowledgments
This work was supported by the Department of General Surgery, Guangzhou First People’s Hospital, Guangzhou Medical University. The study was supported by the Guangdong Provincial Science & Technology Projects (2013B021800041). All the authors designed the study together, performed the experiment together, analyzed the data, and wrote the paper; all the authors approved the final manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shen, F., Cai, WS., Feng, Z. et al. MiR-492 contributes to cell proliferation and cell cycle of human breast cancer cells by suppressing SOX7 expression. Tumor Biol. 36, 1913–1921 (2015). https://doi.org/10.1007/s13277-014-2794-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2794-z