Abstract
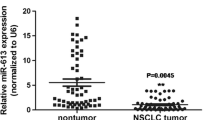
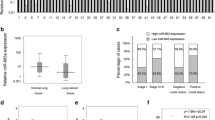
The current study aims to investigate the fuctional role of miRNA-25 in non-small cell lung cancer (NSCLC) cells. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the expression of miR-25 in NSCLC cell lines and 11 pairs of human NSCLC and non-cancerous tissues. The inhibitor of miR-25 was stably transfected into NSCLC cell line A549 cells. Then the effects of downregulating miR-25 on cancer cell proliferation, cell cycle arrest, chemosensitivity to cisplatin, and growth of in vivo xenograft were investigated. Direct regulation of miR-25 on its target gene, cell division cycle 42 (CDC42), was examined by luciferase reporter assay, qRT-PCR and western blot. CDC42 was then upregulated in A549 cells to investigate its effect on miR-25-mediated NSCLC cell proliferation and cell cycle arrest. The expression of miR-25 in NSCLC cells or human tissues was significantly higher than that in normal lung cells or adjacent non-cancerous tissues, respectively. Downregulation of miR-25 markedly inhibited A549 cell proliferation, induced G1 cell cycle arrest, increased cisplatin sensitivity, and suppressed the growth of caner cell xenograft in vivo. CDC42 was confirmed to be the directly regulated by miR-25 in A549 cells. Upregulation of CDC42 in A549 cells rescued the inhibitory effect on proliferation and the G1 cell cycle arrest induced by miR-25 downregulation. Our study demonstrates miR-25, by targeting CDC42, is an important regulator in NSCLC.





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer J Clin. 2012;62:10–29.
Rivera MP. Multimodality therapy in the treatment of lung cancer. Semin Respir Crit Care Med. 2004;25 Suppl 1:3–10.
Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20.
Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–61.
Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–7.
He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33.
Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–80.
Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–79.
Lynam-Lennon N, Maher SG, Reynolds JV. The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos Soc. 2009;84:55–71.
Wang Y, Lee CG. MicroRNA and cancer—focus on apoptosis. J Cell Mol Med. 2009;13:12–23.
Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Ther. 2010;17:523–31.
Nordentoft I, Birkenkamp-Demtroder K, Agerbaek M, Theodorescu D, Ostenfeld MS, Hartmann A, et al. MiRNAs associated with chemo-sensitivity in cell lines and in advanced bladder cancer. BMC Med Genet. 2012;5:40.
Singh S, Chitkara D, Kumar V, Behrman SW, Mahato RI. MiRNA profiling in pancreatic cancer and restoration of chemosensitivity. Cancer Lett. 2013;334:211–20.
Kim CH, Kim HK, Rettig RL, Kim J, Lee ET, Aprelikova O, et al. MiRNA signature associated with outcome of gastric cancer patients following chemotherapy. BMC Med Genet. 2011;4:79.
Zhang H, Zuo Z, Lu X, Wang L, Wang H, Zhu Z. MiR-25 regulates apoptosis by targeting Bim in human ovarian cancer. Oncol Rep. 2012;27:594–8.
Smith AL, Iwanaga R, Drasin DJ, Micalizzi DS, Vartuli RL, Tan AC, et al. The miR-106b-25 cluster targets Smad7, activates TGF-beta signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene. 2012;31:5162–71.
Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, et al. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci. 2009;100:1234–42.
Tan W, Li Y, Lim SG, Tan TM. MiR-106b-25/miR-17-92 clusters: polycistrons with oncogenic roles in hepatocellular carcinoma. World J Gastroenterol: WJG. 2014;20:5962–72.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006.
Landi MT, Zhao Y, Rotunno M, Koshiol J, Liu H, Bergen AW, et al. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin Cancer Res: off J Am Assoc Cancer Res. 2010;16:430–41.
Arias-Romero LE, Chernoff J. Targeting Cdc42 in cancer. Expert Opin Ther Targets. 2013;17:1263–73.
Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23:1415–23.
Zhang JY, Zhang D, Wang EH. Overexpression of small GTPases directly correlates with expression of delta-catenin and their coexpression predicts a poor clinical outcome in nonsmall cell lung cancer. Mol Carcinog. 2013;52:338–47.
Hua KT, Tan CT, Johansson G, Lee JM, Yang PW, Lu HY, et al. N-alpha-acetyltransferase 10 protein suppresses cancer cell metastasis by binding PIX proteins and inhibiting Cdc42/Rac1 activity. Cancer Cell. 2011;19:218–31.
Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, T., Chen, T., Li, Y. et al. Downregulation of miR-25 modulates non-small cell lung cancer cells by targeting CDC42. Tumor Biol. 36, 1903–1911 (2015). https://doi.org/10.1007/s13277-014-2793-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2793-0