Abstract
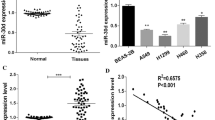
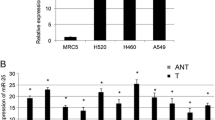
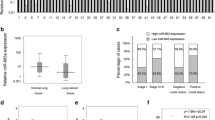
Recent studies have implied that aberration of miR-24 is linked to various human cancers. However, its role in non-small cell lung cancer (NSCLC) remains obscure. Here, we found that miR-24 was significantly upregulated in NSCLC tissues and patients’ serum. High expression of miR-24 in patients’ serum was independently correlated with a shorter overall survival of NSCLC patients. Depletion of miR-24 inhibited cell proliferation and anchorage-independent survival ability in lung cancer cell lines and reduced tumor formation ability in nude mice. Nuclear apoptosis-inducing factor 1 (NAIF1) was identified to be a functional target of miR-24 in the human lung. Next, we observed that the NAIF1 mRNA expression level in NSCLC tissues was suppressed in comparison to that in adjacent normal tissues. Restoration of NAIF1 in lung cancer cell inhibited cell proliferation and anchorage-independent survival ability, which were found to be similar with those from transfecting a miR-24 inhibitor into lung cancer cells. In conclusion, our study demonstrated that miR-24 was upregulated in NSCLC, and suppressing the expression of miR-24 inhibited tumor characteristics. MiR-24 acted as an oncomir, at least partially through regulation of its functional target NAIF1 in NSCLC. MiR-24 may serve as a novel potential biomarker for NSCLC diagnosis and prognosis.





Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103.
He L, He X, Lowe SW, Hannon GJ. microRNAs join the p53 network—another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007;7:819–22.
Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69.
Guo Y, Fu W, Chen H, Shang C, Zhong M. miR-24 functions as a tumor suppressor in Hep2 laryngeal carcinoma cells partly through down-regulation of the S100A8 protein. Oncol Rep. 2012;27:1097–103.
Le HB, Zhu WY, Chen DD, He JY, Huang YY, Liu XG, et al. Evaluation of dynamic change of serum miR-21 and miR-24 in pre- and post-operative lung carcinoma patients. Med Oncol. 2012;29:3190–7.
Haraguchi T, Ozaki Y, Iba H. Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res. 2009;37:e43.
Cao ZA, Daniel D, Hanahan D. Sub-lethal radiation enhances anti-tumor immunotherapy in a transgenic mouse model of pancreatic cancer. BMC Cancer. 2002;2:11.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8.
Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, et al. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148.
Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251:499–505.
Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci U S A. 2008;105:3903–8.
Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L, et al. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–64.
Li J, Song Y, Wang Y, Luo J, Yu W. MicroRNA-148a suppresses epithelial-to-mesenchymal transition by targeting ROCK1 in non-small cell lung cancer cells. Mol Cell Biochem. 2013;380:277–82.
Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA, Wang B, et al. Downregulation of miR-497 promotes tumor growth and angiogenesis by targeting HDGF in non-small cell lung cancer. Biochem Biophys Res Commun. 2013;435:466–71.
Du WW, Fang L, Li M, Yang X, Liang Y, Peng C, et al. MicroRNA miR-24 enhances tumor invasion and metastasis by targeting PTPN9 and PTPRF to promote EGF signaling. J Cell Sci. 2013;126:1440–53.
Chen L, Zhang A, Li Y, Zhang K, Han L, Du W, et al. MiR-24 regulates the proliferation and invasion of glioma by ST7L via beta-catenin/Tcf-4 signaling. Cancer Lett. 2013;329:174–80.
Lin SC, Liu CJ, Lin JA, Chiang WF, Hung PS, Chang KW. miR-24 up-regulation in oral carcinoma: positive association from clinical and in vitro analysis. Oral Oncol. 2010;46:204–8.
Zaidi SK, Dowdy CR, van Wijnen AJ, Lian JB, Raza A, Stein JL, et al. Altered Runx1 subnuclear targeting enhances myeloid cell proliferation and blocks differentiation by activating a miR-24/MKP-7/MAPK network. Cancer Res. 2009;69:8249–55.
Papadimitriou E, Vasilaki E, Vorvis C, Iliopoulos D, Moustakas A, Kardassis D, et al. Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-beta and miR-24: role in epithelial-to-mesenchymal transition. Oncogene. 2012;31:2862–75.
Lv B, Shi T, Wang X, Song Q, Zhang Y, Shen Y, et al. Overexpression of the novel human gene, nuclear apoptosis-inducing factor 1, induces apoptosis. Int J Biochem Cell Biol. 2006;38:671–83.
Luo Q, Zhao M, Zhong J, Ma Y, Deng G, Liu J, et al. NAIF1 is down-regulated in gastric cancer and promotes apoptosis through the caspase-9 pathway in human MKN45 cells. Oncol Rep. 2011;25:1117–23.
Acknowledgments
This work was supported in part by the Harbin Science and Technology Bureau (grant 2012RFQGJ183, Xian Liu).
Conflicts of interest
There are no known conflicts of interest associated with this paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
G. Zhao and L. Liu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
a. Analysis of luciferase activity. A549 cells which have high endogenous miR-24 expression were cotransfected with firefly luciferase reporter containing 3′UTRs of interested genes, and Renilla luciferase expression construct (as an internal control). Luciferase activity was assayed 24 h after transfection. Firefly luciferase values, normalized for Renilla luciferase, are presented. The data represent the mean ± SE of four independent experiments done in duplicates. b. Western blot analysis of KSR2, FGF11, NAIF1, and β-actin in A549 cell extracts transfected with U6-miR-24 TuD plasmid. (TIFF 126 kb)
Rights and permissions
About this article
Cite this article
Zhao, G., Liu, L., Zhao, T. et al. Upregulation of miR-24 promotes cell proliferation by targeting NAIF1 in non-small cell lung cancer. Tumor Biol. 36, 3693–3701 (2015). https://doi.org/10.1007/s13277-014-3008-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-3008-4