Abstract
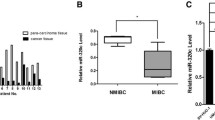
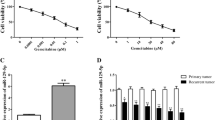
Cancer cells exhibit the ability to metabolise glucose to lactate even under aerobic conditions for energy. This phenomenon is known as the Warburg effect and can be a potential target to kill cancer cells. Several studies have shown evidence for interplay between microRNAs and key metabolic enzyme effecters, which can facilitate the Warburg effect in cancer cells. In the present study, a microRNA sponge forcibly expressed using a lentiviral vector was utilised to knock down miR-21 expression in vitro. qPCR and Western blot assays were performed to evaluate the expression of a regulatory factor related to aerobic glycolysis and the signalling pathway it regulates. In bladder cancer specimens, expression levels of glycolysis-related genes [glucose transporter (GLUT)1, GLUT3, lactic dehydrogenase (LDH)A, LDHB, hexokinase (HK)1, HK2, pyruvate kinase type M (PKM) and hypoxia-inducible factor 1-alpha (HIF-1α)] were higher in tumour tissues than in adjacent tissues, suggesting the role of glycolysis in bladder cancer. miR-21 inhibition in bladder cancer cell lines resulted in reduction in tumour aerobic glycolysis. Decrease in glucose uptake and lactate production was observed upon expression of the miR-21 sponge, which promoted phosphatase and tensin homologue (PTEN) expression, decreased phosphorylated AKT and deactivated mTOR. Furthermore, messenger RNA (mRNA) and protein expression levels of glycolysis-related genes were also lower in miR-21 sponge cells compared to miR-21 control cells. Our findings suggest that miR-21 acts as a molecular switch to regulate aerobic glycolysis in bladder cancer cells via the PTEN/phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway. Blocking miR-21 function can be an effective diagnostic and therapeutic approach either by itself or in combination with existing methods to treat bladder cancer.





Similar content being viewed by others
References
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95.
Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46.
Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34.
Singh PK, Mehla K, Hollingsworth MA, Johnson KR. Regulation of aerobic glycolysis by microRNAs in cancer. Mol Cell Pharmacol. 2011;3:125–34.
Wei Z, Cui L, Mei Z, Liu M, Zhang D. miR-181a mediates metabolic shift in colon cancer cells via the PTEN/AKT pathway. FEBS Lett. 2014;588:1773–9.
Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man Ng R, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer J int du Cancer. 2008;123:251–7.
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3.
Robey RB, Hay N. Is Akt the “Warburg kinase”?—Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31.
Bao L, Yan Y, Xu C, Ji W, Shen S, Xu G, et al. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett. 2013;337:226–36.
Liu J, Zhu H, Yang X, Ge Y, Zhang C, Qin Q, et al. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumour Biol. 2014;35:3975–9.
Tao J, Lu Q, Wu D, Li P, Xu B, Qing W, et al. microRNA-21 modulates cell proliferation and sensitivity to doxorubicin in bladder cancer cells. Oncol Rep. 2011;25:1721–9.
Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282:20534–43.
Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–4.
Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med. 2011;89:221–8.
Zha X, Sun Q, Zhang H. mTOR upregulation of glycolytic enzymes promotes tumor development. Cell Cycle. 2011;10:1015–6.
Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A. 2011;108:4129–34.
Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–20.
Dwarakanath B, Jain V. Targeting glucose metabolism with 2-deoxy-d-glucose for improving cancer therapy. Future Oncol. 2009;5:581–5.
Sottnik JL, Lori JC, Rose BJ, Thamm DH. Glycolysis inhibition by 2-deoxy-d-glucose reverts the metastatic phenotype in vitro and in vivo. Clin Exp Metastasis. 2011;28:865–75.
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58.
Hopkins BD, Fine B, Steinbach N, Dendy M, Rapp Z, Shaw J, et al. A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science. 2013;341:399–402.
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84.
Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502.
Buller CL, Loberg RD, Fan MH, Zhu Q, Park JL, Vesely E, et al. A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression. Am J Phys Cell Physiol. 2008;295:C836–43.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45.
Buller CL, Heilig CW, Brosius 3rd FC. GLUT1 enhances mTOR activity independently of TSC2 and AMPK. Am J Physiol Renal Physiol. 2011;301:F588–96.
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83.
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS One. 2011;6:e19139.
Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–49.
Acknowledgments
This work was supported by the Program for Development of Innovative Research Team of the First Affiliated Hospital of Nanjing Medical University, the Provincial Initiative Program for Excellency Disciplines of Jiangsu Province, the National Natural Science Foundation of China (grant Nos. 81272832 and 81201997), the Natural Science Foundation of Jiangsu Province (grant No. BK2011848), the Six Major Talent Peak Project of Jiangsu Province (grant No. 2011-WS-121), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Provincial Special Program of Medical Science (BL2012027). The funders had no role in study design, data collection and analysis; decision to publish; or preparation of the manuscript.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao Yang, Yidong Cheng and Pengchao Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 37 kb)
Rights and permissions
About this article
Cite this article
Yang, X., Cheng, Y., Li, P. et al. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumor Biol. 36, 383–391 (2015). https://doi.org/10.1007/s13277-014-2617-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2617-2