Abstract
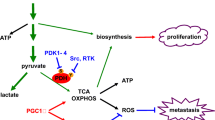
Metastasis is the primary cause of death from many tumors, and novel anti-metastatic therapies are necessary. Recently, we showed that metastatic tumors down-regulate key oxidative phosphorylation (OXPHOS) genes in favor of glycolysis, a further enhancement of the Warburg effect. Therefore, we sought to determine if restriction of glycolysis using 2-deoxy-d-glucose (2DG) would lead to increased utilization of OXPHOS and inhibition of the metastatic phenotype. Noncytotoxic concentrations of 2DG dose-dependently inhibited in vitro migration and invasion in the highly metastatic DLM8-luc-M1 osteosarcoma (OS) cell line, as well as other metastatic human, canine, and murine cancer cells of different histotypes. This was associated with cytoskeletal rearrangement and inhibition of cathepsin L expression. A dose-dependent shift toward OXPHOS was confirmed by demonstrating increased oxygen utilization and decreased lactate production in 2DG treated cells. Finally, 2DG treatment significantly delayed metastasis and prolonged survival in an orthotopic postsurgical OS model. In conclusion, this work suggests that forcing cells away from glycolysis may inhibit key components of the metastatic phenotype, providing a novel avenue for metastasis prevention.






Similar content being viewed by others
References
Bielack SS, Carrle D, Hardes J et al (2008) Bone tumors in adolescents and young adults. Curr Treat Option Oncol 9(1):67–80
Khanna C (2008) Novel targets with potential therapeutic applications in osteosarcoma. Curr Oncol Rep 10(4):350–358
Ramaswamy S, Ross KN, Lander ES et al (2003) A molecular signature of metastasis in primary solid tumors. Nat Genet 33(1):49–54
Ptitsyn AA, Weil MM, Thamm DH (2008) Systems biology approach to identification of biomarkers for metastatic progression in cancer. BMC Bioinform 9(Suppl 9):S8
Chen Y, Cairns R, Papandreou I et al (2009) Oxygen consumption can regulate the growth of tumors, a new perspective on the warburg effect. PloS One 4(9):e7033
Ramanathan A, Wang C, Schreiber SL (2005) Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci USA 102(17):5992–5997
Acebo P, Giner D, Calvo P et al (2009) Cancer abolishes the tissue type-specific differences in the phenotype of energetic metabolism. Transl Oncol 2(3):138–145
Amuthan G, Biswas G, Ananadatheerthavarada HK et al (2002) Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene 21(51):7839–7849
Amuthan G, Biswas G, Zhang SY et al (2001) Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J 20(8):1910–1920
Denhardt DT, Greenberg AH, Egan SE et al (1987) Cysteine proteinase cathepsin L expression correlates closely with the metastatic potential of H-ras-transformed murine fibroblasts. Oncogene 2(1):55–59
Frade R, Rodrigues-Lima F, Huang S et al (1998) Procathepsin-L, a proteinase that cleaves human C3 (the third component of complement), confers high tumorigenic and metastatic properties to human melanoma cells. Cancer Res 58(13):2733–2736
Brown J (1962) Effects of 2-deoxyglucose on carbohydrate metabolism: review of the literature and studies in the rat. Metab Clin Exp 11:1098–1112
Pelicano H, Martin DS, Xu RH et al (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25(34):4633–4646
Geschwind JF, Ko YH, Torbenson MS et al (2002) Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res 62(14):3909–3913
Fulda S, Galluzzi L, Kroemer G (2010) Targeting mitochondria for cancer therapy. Nat Rev 9(6):447–464
Sottnik JL, Duval DL, Ehrhart EJ et al (2010) An orthotopic, postsurgical model of luciferase transfected murine osteosarcoma with spontaneous metastasis. Clin Exp Metastasis 27(3):151–160
Dehn DL, Siegel D, Zafar KS et al (2006) 5-Methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione, a mechanism-based inhibitor of NAD(P)H:quinoneoxidoreductase 1, exhibits activity against human pancreatic cancer in vitro and in vivo. Mol Cancer Ther 5(7):1702–1709
Janeway KA, Walkley CR (2010) Modeling human osteosarcoma in the mouse: from bedside to bench. Bone 47(5):859–865
Souhami RL, Craft AW, Van der Eijken JW et al (1997) Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet 350(9082):911–917
Liu H, Hu YP, Savaraj N et al (2001) Hypersensitization of tumor cells to glycolytic inhibitors. Biochemistry 40(18):5542–5547
Maschek G, Savaraj N, Priebe W et al (2004) 2-Deoxy-d-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64(1):31–34
Garber K (2004) Energy boost: the Warburg effect returns in a new theory of cancer. J Natl Cancer Inst 96(24):1805–1806
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hua Y, Qiu Y, Zhao A et al (2011) Dynamic metabolic transformation in tumor invasion and metastasis in mice with LM-8 osteosarcoma cell transplantation. J Proteome Res 10(8):3513–3521
Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science (New York, NY) 330(6009):1340–1344
Plas DR, Thompson CB (2005) Akt-dependent transformation: there is more to growth than just surviving. Oncogene 24(50):7435–7442
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY) 324(5930):1029–1033
Biswas G, Tang W, Sondheimer N et al (2008) A distinctive physiological role for IkappaBbeta in the propagation of mitochondrial respiratory stress signaling. J Biol Chem 283(18):12586–12594
Guha M, Srinivasan S, Biswas G et al (2007) Activation of a novel calcineurin-mediated insulin-like growth factor-1 receptor pathway, altered metabolism, and tumor cell invasion in cells subjected to mitochondrial respiratory stress. J Biol Chem 282(19):14536–14546
Cuezva JM, Krajewska M, de Heredia ML et al (2002) The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res 62(22):6674–6681
Willers IM, Isidoro A, Ortega AD et al (2010) Selective inhibition of beta-F1-ATPase mRNA translation in human tumours. Biochem J 426(3):319–326
Simons AL, Fath MA, Mattson DM et al (2007) Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-d-glucose correlates with increased 18F-FDG uptake as determined by PET imaging. Int J Radiat Oncol Biol Phys 69(4):1222–1230
Kurtoglu M, Maher JC, Lampidis TJ (2007) Differential toxic mechanisms of 2-deoxy-d-glucose versus 2-fluorodeoxy-d-glucose in hypoxic and normoxic tumor cells. Antioxid Redox Signal 9(9):1383–1390
Lampidis TJ, Kurtoglu M, Maher JC et al (2006) Efficacy of 2-halogen substituted d-glucose analogs in blocking glycolysis and killing “hypoxic tumor cells”. Cancer Chemother Pharmacol 58(6):725–734
Maher JC, Savaraj N, Priebe W et al (2005) Differential sensitivity to 2-deoxy-d-glucose between two pancreatic cell lines correlates with GLUT-1 expression. Pancreas 30(2):e34–e39
Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ et al (2009) HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem 9(9):1084–1101
Minor RK, Smith DL Jr, Sossong AM et al (2010) Chronic ingestion of 2-deoxy-d-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol 243(3):332–339
Dwarakanath BS, Singh D, Banerji AK et al (2009) Clinical studies for improving radiotherapy with 2-deoxy-d-glucose: present status and future prospects. J Cancer Res Ther 5(Suppl 1):S21–S26
Stein M, Lin H, Jeyamohan C et al (2010) Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 70(13):1388–1394
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13(6):472–482
Boutros J, Almasan A (2009) Combining 2-deoxy-d-glucose with electron transport chain blockers: a double-edged sword. Cancer Biol Ther 8(13):1237–1238
Fath MA, Diers AR, Aykin-Burns N et al (2009) Mitochondrial electron transport chain blockers enhance 2-deoxy-d-glucose induced oxidative stress and cell killing in human colon carcinoma cells. Cancer Biol Ther 8(13):1228–1236
Ben Sahra I, Laurent K, Giuliano S et al (2010) Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 70(6):2465–2475
Sahra IB, Tanti JF, Bost F (2010) The combination of metformin and 2-deoxyglucose inhibits autophagy and induces AMPK dependent apoptosis in prostate cancer cells. Autophagy 6(5). doi:10.1158/0008-5472.CAN-09-2782
Zhao Y, Liu H, Liu Z et al (2011) Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res 71(13):4585–4597
Kurtoglu M, Gao N, Shang J et al (2007) Under normoxia, 2-deoxy-d-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther 6(11):3049–3058
Maher JC, Wangpaichitr M, Savaraj N et al (2007) Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-d-glucose. Mol Cancer Ther 6(2):732–741
Langbein S, Frederiks WM, Zur Hausen A et al (2008) Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer 122(11):2422–2428
Krockenberger M, Engel JB, Schmidt M et al (2010) Expression of transketolase-like 1 protein (TKTL1) in human endometrial cancer. Anticancer Res 30(5):1653–1659
Sun W, Liu Y, Glazer CA et al (2010) TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res 16(3):857–866
Volker HU, Hagemann C, Coy J et al (2008) Expression of transketolase-like 1 and activation of Akt in grade IV glioblastomas compared with grades II and III astrocyticgliomas. Am J Clin Pathol 130(1):50–57
Xu X, Zur Hausen A, Coy JF et al (2009) Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer 124(6):1330–1337
Xi H, Kurtoglu M, Liu H et al (2011) 2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharmacol 67(4):899–910
Acknowledgments
The authors would like to thank Jenette Shoeneman, Laura Chubb, Dr. Scott Hafeman, and Dr. Jessica Cantrell for assistance with the animal experiments, Polly Webb for assistance with lactate measurements, Dr. David Siegel (University of Colorado Health Sciences Center) for his help in performing the oxygen utilization experiments, and Dr. Daniel Gustafson for critical manuscript review. This work was supported by the Colorado State University Supercluster and the estate of Mr. Jeffery Harbers.
Conflict of interest
The authors declare they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2011_9417_MOESM1_ESM.tiff
Supplemental Fig. 1: 2DG does not significantly impact clonogenic cell growth. DLM8-Luc-M1 cells were treated with varying concentrations of 2DG for 24 h, followed by plating in 10 cm dishes. Colonies were stained with crystal violet and counted manually. There was no significant difference in colony formation upon 2DG exposure. Error bars indicate standard deviation. (TIFF 5691 kb)
10585_2011_9417_MOESM2_ESM.tif
Supplemental Fig. 2: 2DG does not significantly impact cell luminosity. a DLM8-luc-M1 cells were treated with varying concentrations of 2DG before the addition of luciferin and determination of photon flux. Each condition was analyzed in triplicate and mean ± SD depicted. Mice were challenged subcutaneously with DLM8-luc-M1 tumor cells. Once the mean tumor diameter was 5 mm, treatment with 2DG was initiated. b No significant inhibition of tumor cross-sectional area was observed. Tumor bioluminescence was measured to determine if 2DG inhibited photon flux. c There was no significant difference in tumor bioluminescence when normalized by tumor cross-sectional area. (TIFF 5465 kb)
Rights and permissions
About this article
Cite this article
Sottnik, J.L., Lori, J.C., Rose, B.J. et al. Glycolysis inhibition by 2-deoxy-d-glucose reverts the metastatic phenotype in vitro and in vivo. Clin Exp Metastasis 28, 865–875 (2011). https://doi.org/10.1007/s10585-011-9417-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9417-5