Abstract
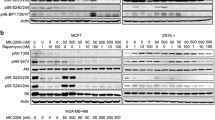
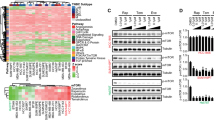
Blockade of mammalian target of rapamycin (mTOR) is a promising area in breast cancer therapy. However, in clinical trials, objective response rate with mTOR inhibitor monotherapy in breast cancer was modest. Biomarker studies designed to identify the responders of rapalogs are of increasing interest. We validated p27KIP1 expression levels as a candidate predictive biomarker of response to rapalogs. We also analyzed the correlation between rapamycin activity and p27KIP1 expression in the primary breast cancer cells and the patient-derived breast tumor xenograft models. The cells isolated from the breast tumor tissues expressing high levels of p27KIP1 were sensitive to rapamycin, whereas the cells from the tissues expressing low levels of p27KIP1 exhibited resistance to rapamycin. The correlation between p27KIP1 expression and rapamycin antitumor activity was also observed in the patient-derived breast tumor xenograft models. Moreover, we also found rapamycin significantly decreased phosphorylated p70S6K1 and phosphorylated 4EBP1 in both samples. It seemed that the different sensitivity of tumor cells to rapamycin did not owe to its different potency against mTOR activity. We further propose p27KIP1 expression level may be also a candidate predictive biomarker of rapalogs for breast cancer therapy, which requires additional clinical validation.




Similar content being viewed by others
References
Awada A, Cardoso F, Fontaine C, Dirix L, De Greve J, Sotiriou C, et al. The oral mTOR inhibitor rad001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91.
Zhang X, Claerhout S, Prat A, Dobrolecki LE, Petrovic I, Lai Q, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013;73:4885–97.
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7.
Martin LA, Andre F, Campone M, Bachelot T, Jerusalem G. mTOR inhibitors in advanced breast cancer: ready for prime time? Cancer Treat Rev. 2013;39:742–52.
Vinayak S, Carlson RW: mTOR inhibitors in the treatment of breast cancer. Oncology (Williston Park) 2013;27:38–44, 46, 48 passim.
Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93.
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56.
Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–22.
Wan X, Helman LJ. The biology behind mTOR inhibition in sarcoma. Oncologist. 2007;12:1007–18.
Chen G, Yang N, Wang X, Zheng SY, Chen Y, Tong LJ, et al. Identification of p27/kip1 expression level as a candidate biomarker of response to rapalogs therapy in human cancer. J Mol Med (Berl). 2010;88:941–52.
Chu IM, Hengst L, Slingerland JM. The CDK inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67.
Tigli H, Buyru N, Dalay N. Molecular analysis of the p27/kip1 gene in breast cancer. Mol Diagn J Devoted Underst Hum Dis Clin Appl Mol Biol. 2005;9:17–21.
Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, et al. Expression of cell-cycle regulators p27kip1 and cyclin e, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–5.
Filipits M, Rudas M, Heinzl H, Jakesz R, Kubista E, Lax S, et al. Colorectal cancer study G: low p27 expression predicts early relapse and death in postmenopausal hormone receptor-positive breast cancer patients receiving adjuvant tamoxifen therapy. Cli Can Res Off J Am Assoc Cancer Res. 2009;15:5888–94.
Oka K, Suzuki Y, Nakano T. Expression of p27 and p53 in cervical squamous cell carcinoma patients treated with radiotherapy alone: radiotherapeutic effect and prognosis. Cancer. 2000;88:2766–73.
Oshita F, Kameda Y, Nishio K, Tanaka G, Yamada K, Nomura I, et al. Increased expression levels of cyclin-dependent kinase inhibitor p27 correlate with good responses to platinum-based chemotherapy in non-small cell lung cancer. Oncol Rep. 2000;7:491–5.
Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–50.
Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, et al. Interleukin-2-mediated elimination of the p27kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3.
Woltman AM, van der Kooij SW, Coffer PJ, Offringa R, Daha MR, van Kooten C. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27kip1 expression. Blood. 2003;101:1439–45.
Dalvai M, Schubart K, Besson A, Matthias P. Oct1 is required for mTOR-induced G1 cell cycle arrest via the control of p27(kip1) expression. Cell Cycle. 2010;9:3933–44.
Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–11.
Acknowledgments
This work was supported by Zhejiang Provincial Natural Science Foundation of China (no. LY12H31001, Y2110474), National Natural Science Foundation of China (no. 81201530), and Foundation of Zhejiang Educational Committee (no. Y201121896).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao-Fei Ding and Dong-Qing Yin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ding, XF., Yin, DQ., Chen, Q. et al. Validation of p27KIP1 expression levels as a candidate predictive biomarker of response to rapalogs in patient-derived breast tumor xenografts. Tumor Biol. 36, 1463–1469 (2015). https://doi.org/10.1007/s13277-014-2580-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2580-y