Abstract
The association between methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and breast cancer risk in the Chinese population has been widely reported, but results were inconsistent. In order to derive a more precise estimation of the relationship, a meta-analysis was performed. Eligible articles were identified through search of databases including Medline, PubMed, Web of Science, Embase, Chinese Biomedical Literature Database (CBM, Chinese), China National Knowledge Infrastructure (CNKI, Chinese), and Wangfang Database (Chinese). The association between the MTHFR polymorphism and breast cancer risk was conducted using odds ratios (ORs) and 95 % confidence intervals (95 % CIs). Finally, a total of 22 studies with 6,103 cases and 7,913 controls were included in our meta-analysis: 13 studies with 3,273 cases and 4,419 controls for C677T polymorphism and 9 studies with 2,830 cases and 3,494 controls for A1298C polymorphism. With regard to C677T polymorphism, significant association was found with breast cancer risk under three models (T vs. C: OR = 1.12, 95 % CI = 1.02–1.23, P = 0.015; TT vs. CC: OR = 1.35, 95 % CI = 1.10–1.67, P = 0.005; TT vs. CC/CT: OR = 1.37, 95 % CI = 1.11–1.70, P = 0.004). There was no significant association found between A1298C polymorphism and breast cancer risk under all genetic models (C vs. A: OR = 0.96, 95 % CI = 0.89–1.03, P = 0.268; CC vs. AA: OR = 0.98, 95 % CI = 0.77–1.26, P = 0.899; AC vs. AA: OR = 0.95, 95 % CI = 0.88–1.02, P = 0.174; CC vs. AC/AA: OR = 1.00, 95 % CI = 0.78–1.28, P = 0.996, CC/AC vs. AA: OR = 0.96, 95 % CI = 0.89–1.02, P = 0.196). In summary, during this meta-analysis, we found that MTHFR C677T polymorphism was significantly associated with breast cancer risk in the Chinese population. Meanwhile, MTHFR A1298C polymorphism was not associated with breast cancer risk in the Chinese population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most prevalent invasive cancers and the second leading global cause of cancer-related deaths among women, in both developed and developing countries, which has become a major public health challenge [1, 2]. Global breast cancer incidence has been increasing by more than one million new cases every year; the incidence is significantly higher in developed countries than in developing countries [3]. Breast cancer increased significantly in China, especially in Beijing and Shanghai; it increased by 23 and 31 % within 10 years, respectively. It is close to the levels of western high-prevalence countries of breast cancer [4]. The mechanism of breast carcinogenesis is still not fully understood. Some factors such as familial history of the disease, age of menarche and menopause, diet, reproductive history, high estrogen exposure, and genetic factors are considered to be risk factors for breast cancer. There are studies suggesting that the effect determined by low-penetrance genes may provide a plausible explanation for breast cancer susceptibility, and in recent years, several common low-penetrance genes have been identified as potential breast cancer susceptibility genes [5–9].
As one of the important low-penetrance genes, 5,10-methylenetetrahydrofolate reductase (MTHFR) encodes a critical enzyme for intracellular folate homeostasis and metabolism, which catalyzes the conversion of 5,10-methylenetetrahydrofolate (5,10-methylene-THF) to 5-methyltetrahydrofolate (5-methylene-THF), and it is thought to influence DNA methylation and nucleic acid synthesis [10–12]. The MTHFR polymorphisms were considered to be associated with breast cancer susceptibility [13, 14].
The C677T (rs1801133, Ala222Val) and A1298C (rs1801131, Glu429Ala) are two common polymorphisms of MTHFR genes. C677T is in exon 4 at nucleotide 677, which is associated with the decrease of MTHFR activity and increased the level of homocysteine and altered the distribution of folate, while A1298C (rs1801131, Glu429Ala) is in exon 7 at nucleotide 1298, which is also related to the reduction of MTHFR activity but at a lower degree compared to C677T [15–17]. A number of studies indicate that C677T and A1298C polymorphisms in the MTHFR gene were involved in the etiology of breast cancer among the Chinese population [18–31]. However, the results from those studies remain conflicting.
In order to get more accurate results, we performed a meta-analysis. In this study, we intend to explore the possible association between two common variants of the MTHFR gene, C677T and A1298C, and breast cancer risk in Chinese patients. To our knowledge, this is the most comprehensive meta-analysis conducted to date with respect to the association between MTHFR gene polymorphisms and breast cancer risk among the Chinese population.
Materials and methods
Search strategy
A comprehensive search strategy was conducted towards the electronic databases including Medline, PubMed, Web of Science, Embase, Chinese Biomedical Literature Database (CBM, Chinese), China National Knowledge Infrastructure (CNKI, Chinese), and Wangfang Database (Chinese) with keywords “breast cancer,” “breast neoplasm,” “methylenetetrahydrofolate reductase,” “MTHFR,” “polymorphism,” and “variant” for all studies searched on the Chinese people, and there were no limitations to the language of publications. Additional studies were identified by a hand search of the references of original studies; review articles were also examined to find additional eligible studies.
Inclusion and exclusion criteria
Eligible studies had to meet all of the following criteria: (a) the publication was a case–control study referring to the association between MTHFR polymorphisms and breast cancer in Chinese people; (b) the articles must offer the sample size, distribution of alleles, genotypes, or other information for estimating the odds ratio (OR) and 95 % confidence interval (CI); (c) when multiple publications reported on the same or overlapping data, we used the most recent or largest population; and (d) the studies were published. The following exclusion criteria were used for excluding studies: (a) not a case–control study, (b) studies that contained duplicate data, (c) no usable data reported, and (d) case reports or reviews.
Data extraction
Data were carefully extracted by two authors independently from each study based on the inclusion criteria mentioned above. If conflicting evaluations were encountered, an agreement was reached following a discussion; if agreement could not be reached, then a third author was consulted to resolve the debate. The following information were extracted: (a) the name of the first author, (b) year of publication, (c) city of origin, (d) the language of each study, (e) genotyping methods, (f) source of the control group, and (g) distribution of genotypes in case and control groups. We also evaluated whether the genotype distributions were in Hardy–Weinberg equilibrium.
Statistical analysis
The strength of association between the MTHFR C677T polymorphisms and breast cancer risk was evaluated by OR and 95 % CI according to allele contrast (T vs. C), homozygote (TT vs. CC), heterozygote (TC vs. CC), recessive (TT vs. TC/ CC), and dominant (TT/TC vs. CC) models, while the possible association between the MTHFR A1298C polymorphism and breast cancer risk was assessed by OR and 95 % CI according to allele contrast (C vs. A), homozygote (CC vs. AA), heterozygote (CA vs. AA), dominant (CC/AC vs. AA), and recessive (CC vs. AC/AA) models, respectively. The heterogeneity was assessed by a chi-square-based Q statistic test. The effect of heterogeneity was quantified by using the I 2 value as well as P value [32]. If the I 2 value >50 % or P < 0.10, then that suggests that obvious heterogeneity existed. ORs were pooled by a random effects model (the DerSimonian and Laird method) [33]. Otherwise, a fixed effects model (the Mantel–Haenszel method) was used [34].
The Hardy–Weinberg equilibrium [35] of controls was tested by using a professional web-based program (http://ihg2.helmholtz-muenchen.de/cgibin/hw/hwa1.pl). If P > 0.05, then it suggests that the controls followed the Hardy–Weinberg equilibrium (HWE) balance. Sensitivity analysis was performed to assess the stability of the results. A single study involved in the meta-analysis was removed each time to reflect the influence of the individual data set to the pooled ORs [36]. When the Hardy–Weinberg equilibrium disequilibrium existed (P < 0.05 was considered statistically significant), the sensitivity analysis was also conducted. Possible publication bias was assessed by Egger's test (P < 0.05 was considered representative of statistically significant publication bias) [37] and visual observation of funnel plot [38]. All statistical tests were performed with STATA software (version 9.2, Stata Corp.). A P value of less than 0.05 for any test or model was considered to be statistically significant.
Results
Search results and study characteristics
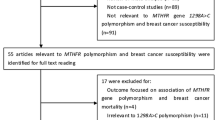
After careful examination according to the inclusion criteria, a total of 22 studies [18–31] with 6,103 cases and 7,913 controls were included in our meta-analysis: 13 studies with 3,273 cases and 4419 controls for C677T polymorphism (Table 1) and 9 studies with 2,830 cases and 3,494 controls for A1298C polymorphism (Table 2). The genotype distributions in the controls of all studies were consistent with HWE (all P > 0.05).
Meta-analysis results
The main results of this meta-analysis and the heterogeneity test were shown in Tables 3 and 4. With regard to C677T polymorphism, significant association was found with breast cancer risk under three models (T vs. C: OR = 1.12, 95 % CI = 1.02–1.23, P = 0.015; TT vs. CC: OR = 1.35, 95 % CI = 1.10–1.67, P = 0.005 (Fig. 2a); TT vs. CC/CT: OR = 1.37, 95 % CI = 1.11–1.70, P = 0.004 (Fig. 2c)). In the heterozygote model (TC vs. CC: OR = 1.01, 95 % CI = 0.96–1.06, P = 0.659) and dominant model (TT/TC vs. CC: OR = 1.06, 95 % CI = 0.99–1.1, P = 0.087 (Fig. 2b), no association was found between C677T polymorphism and breast cancer risk. There was no significant association found between A1298C polymorphism and breast cancer risk under all genetic models (C vs. A: OR = 0.96, 95 % CI = 0.89–1.03, P = 0.268; CC vs. AA: OR = 0.98, 95 % CI = 0.77–1.26, P = 0.899; AC vs. AA: OR = 0.95, 95 % CI = 0.88–1.02, P = 0.174; CC vs. AC/AA: OR = 1.00, 95 % CI = 0.78–1.28, P = 0.996 (Fig. 1a); CC/AC vs. AA: OR = 0.96, 95 % CI = 0.89–1.02, P = 0.196).
a The forest plot describing the meta-analysis under the recessive model for the association between MTHFR A1298C polymorphism and the risk of breast cancer in the Chinese population (CC vs. CA + AA). b Begg funnel plot for publication bias test for the association between MTHFR A1298C polymorphism and the risk of breast cancer under the recessive model (CC vs. CA/AA). Each point represents a separate study for the indicated association. Log [OR], natural logarithm of OR. Horizontal line means effect size
Sensitivity analysis
Sensitivity analyses were conducted to determine whether modification of the inclusion criteria of the meta-analysis affected the final results. The statistical significance of the results was not altered when any single study was omitted, confirming the stability of the results (data not shown). So, results of the sensitivity analyses suggest that the data in our meta-analysis are relatively stable and credible.
Publication bias
Funnel plot and Egger's test were performed to assess the publication bias. Funnel plot is relatively straightforward to observe whether the publication bias is present, and Egger's test was used to provide statistical evidence of symmetries of the plots. As shown in Fig. 1b (C677T polymorphism) and Fig. 2d (A1298C polymorphism), the shape of the funnel plot did not show obvious asymmetry. Similarly, the results of Egger's test show that no publication bias was found too (all P > 0.05, data not shown).
a The forest plot describing the meta-analysis under the homozygous model for the association between MTHFR C677T polymorphism and the risk of breast cancer in the Chinese population (TT vs. CC). b The forest plot describing the meta-analysis under the dominance model for the association between MTHFR C677T polymorphism and the risk of breast cancer in the Chinese population (TT/TC vs. CC). c The forest plot describing the meta-analysis under the recessive model for the association between MTHFR C677T polymorphism and the risk of breast cancer in the Chinese population (TT vs. TC/CC). d Begg funnel plot for publication bias test for the association between MTHFR C677T polymorphism and the risk of breast cancer under the heterozygous model (TC vs. CC). Each point represents a separate study for the indicated association. Log [OR], natural logarithm of OR. Horizontal line means effect size
Discussion
Breast cancer is one of the most common malignant tumors and leading causes of cancer-related death among females in the world, and it is a threat to women's health. In China, incidence showed a clear upward trend, especially in urban areas. Breast cancer incidence and mortality accounted for the top three most common female malignancy in China. Many candidate genes have been reported to be involved in breast cancer susceptibility, including MTHFR, CYP19 [39], CASP8 [40], GSM1 [7], hOGG1 [41], and so on. MTHFR is one of the primary candidate genes concerning the alteration of MTHFR enzyme activity which may influence the general balance between DNA synthesis, repair, and methylation processes [15, 16, 42]. A series of studies have investigated the association between the MTHFR polymorphisms and breast cancer susceptibility in the Chinese people, but got controversial or inconclusive results.
Meta-analysis is a powerful tool for analyzing cumulative data of studies wherein the individual sample sizes are small and the disease can be easily masked by other genetic and environmental factors [43, 44]. A meta-analysis potentially investigates a large number of individuals and can estimate the effect of a genetic factor on the risk of the disease [2]. The present meta-analysis, including 22 studies with 6,103 cases and 7,913 controls, explored the association between the MTHFR C677T and A1298C polymorphisms and breast cancer risk. Our results indicate that the MTHFR A1298C polymorphism is not associated with breast cancer development in the Chinese population, but a strong association between MTHFR C677T polymorphism and breast cancer risk was found, indicating that potentially functional MTHFR C677T polymorphism may play a low-penetrance role in the development of breast cancer.
Although comprehensive analysis was conducted to show the association between MTHFR polymorphism and risk of breast cancer, there are still some limitations that should be acknowledged. Firstly, the number of studies and the number of samples included in the meta-analysis were relatively small. Secondly, the controls were not uniformly defined. Some studies used controls that were population-based, while others used hospital-based controls, which may not be representative of the general population. Thirdly, our results were based on unadjusted estimates, while a more precise analysis should be conducted if individual data were available, which would allow for the adjustment by other co-variants including age, menopausal status, obesity, environmental factors, and lifestyle.
Despite the limitations above, our meta-analysis also had several advantages: First, a meta-analysis of the association between MTHFR polymorphism and breast cancer risk is statistically more powerful than any other single study. Second, a strict search strategy which combined computer-assisted search and manual search makes the eligible studies to be included as much as possible. Third, the quality of case–control studies included in the meta-analysis met our inclusion criteria and was satisfactory, and the sensitivity analysis and publication bias analysis indicated that the results of our meta-analysis are stable, credible, and convincing.
Conclusion
In summary, during this meta-analysis, we found that the MTHFR C677T polymorphism was significantly associated with breast cancer risk in the Chinese population. Meanwhile, the MTHFR A1298C polymorphism was not associated with breast cancer risk in the Chinese population. Considering the limited sample size and ethnicities included in the meta-analysis, further large-scale and well-designed studies are needed to confirm our results. Moreover, gene–gene and gene–environment interactions should also be considered in a future analysis.
References
Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–83.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Sturgeon SR, Schairer C, Grauman D, El Ghormli L, Devesa S. Trends in breast cancer mortality rates by region of the United States, 1950–1999. Cancer Causes Control. 2004;15:987–95.
Fang Q, Qiong W, Zhang L, Xiaoli M. Analysis of breast cancer epidemic. Chin J Soc Med. 2012;29:333–5.
Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6:213–8.
Dumitrescu RG, Cotarla I. Understanding breast cancer risk—where do we stand in 2005? J Cell Mol Med. 2005;9:208–21.
Qiu LX, Yuan H, Yu KD, Mao C, Chen B, Zhan P, et al. Glutathione S-transferase M1 polymorphism and breast cancer susceptibility: a meta-analysis involving 46,281 subjects. Breast Cancer Res Treat. 2010;121:703–8.
Qiu LX, Yao L, Mao C, Chen B, Zhan P, Xue K, et al. TGFB1 L10P polymorphism is associated with breast cancer susceptibility: evidence from a meta-analysis involving 47,817 subjects. Breast Cancer Res Treat. 2010;123:563–7.
Qiu LX, Yao L, Xue K, Zhang J, Mao C, Chen B, et al. BRCA2 N372H polymorphism and breast cancer susceptibility: a meta-analysis involving 44,903 subjects. Breast Cancer Res Treat. 2010;123:487–90.
Rosenblatt DS. Methylenetetrahydrofolate reductase. Clin Invest Med. 2001;24:56–9.
Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71:121–38.
Lucock M. Is folic acid the ultimate functional food component for disease prevention? BMJ. 2004;328:211–4.
Diakite B, Tazzite A, Hamzi K, Jouhadi H, Nadifi S. Methylenetetrahydrofolate reductase C677T polymorphism and breast cancer risk in moroccan women. Afr Health Sci. 2012;12:204–9.
de Cassia Carvalho Barbosa R, da Costa DM, Cordeiro DE, Vieira AP, Rabenhorst SH. Interaction of MTHFR C677T and A1298C, and MTR A2756G gene polymorphisms in breast cancer risk in a population in northeast Brazil. Anticancer Res. 2012;32:4805–11.
Taioli E, Garza MA, Ahn YO, Bishop DT, Bost J, Budai B, et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and colorectal cancer: a HuGE-GSEC review. Am J Epidemiol. 2009;170:1207–21.
Boccia S, Hung R, Ricciardi G, Gianfagna F, Ebert MP, Fang JY, et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol. 2008;167:505–16.
De Mattia E, Toffoli G. C677T and A1298C MTHFR polymorphisms, a challenge for antifolate and fluoropyrimidine-based therapy personalisation. Eur J Cancer. 2009;45:1333–51.
Wu XY, Ni J, Xu WJ, Zhou T, Wang X. Interactions between MTHFR C677T-A1298C variants and folic acid deficiency affect breast cancer risk in a Chinese population. Asian Pac J Cancer Prev. 2012;13:2199–206.
Gao CM, Tang JH, Cao HX, Ding JH, Wu JZ, Wang J, et al. MTHFR polymorphisms, dietary folate intake and breast cancer risk in Chinese women. J Hum Genet. 2009;54:414–8.
Chou YC, Wu MH, Yu JC, Lee MS, Yang T, Shih HL, et al. Genetic polymorphisms of the methylenetetrahydrofolate reductase gene, plasma folate levels and breast cancer susceptibility: a case–control study in Taiwan. Carcinogenesis. 2006;27:2295–300.
Shrubsole MJ, Gao YT, Cai Q, Shu XO, Dai Q, Hebert JR, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2004;13:190–6.
Inoue M, Robien K, Wang R, Van Den Berg DJ, Koh WP, Yu MC. Green tea intake, MTHFR/TYMS genotype and breast cancer risk: the Singapore Chinese Health Study. Carcinogenesis. 2008;29:1967–72.
Yu CP, Wu MH, Chou YC, Yang T, You SL, Chen CJ, et al. Breast cancer risk associated with multigenotypic polymorphisms in folate-metabolizing genes: a nested case–control study in Taiwan. Anticancer Res. 2007;27:1727–32.
Lin WY, Chou YC, Wu MH, Huang HB, Jeng YL, Wu CC, et al. The MTHFR C677T polymorphism, estrogen exposure and breast cancer risk: a nested case–control study in Taiwan. Anticancer Res. 2004;24:3863–8.
Wu Y, Yuan X, Zheng H, Chu Yanhui, Zhang Jiaying. Northeast methylenetetrahydrofolate reductase gene c677t single nucleotide polymorphisms and susceptibility to breast cancer research. Modern Oncology 2010;18:2375–2378.
Yuan H, Xu X, Wang Z. Mthfrc677t Gene polymorphism and breast cancer. Mudanjiang Med Coll. 2009;30:2–4.
Hua Z, Wang Y, Ni J, Ge F, Zou T. Serum folate, vitamin b12 concentration and mthfr, ms gene polymorphism associated with risk of breast cancer research. Mod Oncol. 2011;19:428–31.
Kan X, Zou T, Wu X, Wang X. Yunnan methylenetetrahydrofolate reductase gene polymorphism associated with breast cancer susceptibility. Cancer Res. 2007;34:716–8.
Li W, Shengqiang C. . Mthfr c 677 t gene polymorphism associated with breast cancer. Practical. J Med. 2009;25:2031–3.
Lin J, Chen S, Li W. Mthfr C677T and a1298c gene polymorphisms associated with breast cancer. Prelim Stud Mod Hosp. 2010;10:15–7.
Qi J, Miao X, Tan W, Yu C-y, Liang G, Lvwen F, et al. Methylenetetrahydrofolate reductase gene single nucleotide polymorphisms and breast cancer risk. Chin J Oncol. 2004;26:287–9.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Zamora-Ros R, Rothwell JA, Scalbert A, Knaze V, Romieu I, Slimani N, et al. Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (epic) study. Br J Nutr. 2013;14:1–12.
Tobias A, Campbell MJ. Modelling influenza epidemics in the relation between black smoke and total mortality. A sensitivity analysis. J Epidemiol Community Health. 1999;53:583–4.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Ma X, Qi X, Chen C, Lin H, Xiong H, Li Y, et al. Association between CYP19 polymorphisms and breast cancer risk: results from 10,592 cases and 11,720 controls. Breast Cancer Res Treat. 2010;122:495–501.
Sergentanis TN, Economopoulos KP. Association of two CASP8 polymorphisms with breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;120:229–34.
Yuan W, Xu L, Feng Y, Yang Y, Chen W, Wang J, et al. The hoGG1 Ser326Cys polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;122:835–42.
Macis D, Maisonneuve P, Johansson H, Bonanni B, Botteri E, Iodice S, et al. Methylenetetrahydrofolate reductase (MTHFR) and breast cancer risk: a nested-case–control study and a pooled meta-analysis. Breast Cancer Res Treat. 2007;106:263–71.
Fujisawa T, Ikegami H, Kawaguchi Y, Ogihara T. Meta-analysis of the association of Trp64Arg polymorphism of beta 3-adrenergic receptor gene with body mass index. J Clin Endocrinol Metab. 1998;83:2441–4.
Liwei L, Chunyu L, Ruifa H. Association between manganese superoxide dismutase gene polymorphism and risk of prostate cancer: a meta-analysis. Urology. 2009;74:884–8.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hongjie Liang and Yulan Yan contributed equally to this work, so they should be considered as the co-first authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Liang, H., Yan, Y., Li, T. et al. Methylenetetrahydrofolate reductase polymorphisms and breast cancer risk in Chinese population: a meta-analysis of 22 case–control studies. Tumor Biol. 35, 1695–1701 (2014). https://doi.org/10.1007/s13277-013-1234-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1234-9