Abstract
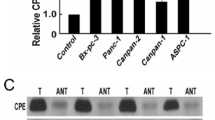
Lymph node (LN) metastasis is one of the most important risk factors for the prognosis of pancreatic cancer. This study aimed to identify novel LN metastasis-associated markers and therapeutic targets for pancreatic and gallbladder cancers. DNA microarray analysis was carried out to identify genes differentially expressed between 17 pancreatic cancer tissues with LN metastasis and 17 pancreatic cancer tissues without LN metastasis. The expression of LZIC, FXR, SCAMP1, and SULT1E1 is significantly higher in pancreatic cancer tissues with LN metastasis than in pancreatic cancer tissues without LN metastasis. We recently reported that FXR plays an important role in LN metastasis of pancreatic cancer, and in this study, we selected the secretory carrier membrane protein 1 (SCAMP1) gene. To determine that function of the SCAMP1 gene, we examined the effects of SCAMP1 knockdown on pancreatic and gallbladder cancer proliferation, migration, and invasion using SCAMP1 small interfering RNA (siRNA) and the activity of vascular endothelial growth factor (VEGF) was measured by enzyme-linked immunosorbent assay. SCAMP1 overexpression is associated with LN metastasis in pancreatic cancer patients. The siRNA-mediated downregulation of SCAMP1 resulted in a marked reduction in cell migration and invasion, but not proliferation in MIA-PaCa2, PANC-1, TGBC-1, and TGBC-2 cells. In addition, downregulation of SCAMP1 inhibited VEGF levels of conditioned medium from SCAMP1 siRNA-transfected cells. These results suggest that downregulation of SCAMP1 could be a potential therapeutic target for patients with pancreatic and gallbladder cancer.






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics. CA Cancer J Clin. 2006;56(2):106–30.
Mao C, Domenico DR, Kim K, Hanson DJ, Howard JM. Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma. Findings of 154 autopsies. Arch Surg. 1995;130:125–34.
Maeda S, Shinchi H, Kurahara H, Mataki Y, Noma H, Maemura K, et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br J Cancer. 2007;97:405–11.
Andren-Sandberg A. Diagnosis and management of gallbladder cancer. N Am J Med Sci. 2012;4(7):293–9.
Lee JY, Lee KT, Lee JK, Lee KH, Jang KT, Heo JS, et al. Farnesoid X receptor, overexpressed in pancreatic cancer with lymph node metastasis promotes cell migration and invasion. Br J Cancer. 2011;104(6):1027–37.
Brand SH, Laurie SM, Mixon MB, Castle JD. Secretory carrier membrane proteins 31–35 define a common protein composition among secretory carrier membranes. J Biol Chem. 1991;266:18949–57.
Cameron RS, Cameron PL, Castle JD. A common spectrum of polypeptides occurs in secretion granule membranes of different exocrine glands. J Cell Biol. 1986;103:1299–313.
Brand SH, Castle JD. SCAMP 37, a new marker within the general cell surface recycling system. EMBO J. 1993;12:3753–61.
Laurie SM, Cain CC, Lienhard GE, Castle JD. The glucose transporter GluT4 and secretory carrier membrane proteins (SCAMPs) colocalize in rat adipocytes and partially segregate during insulin stimulation. J Biol Chem. 1993;268:19110–7.
Liao H, Zhang J, Shestopal S, Szabo G, Castle A, Castle D. Nonredundant function of secretory carrier membrane protein isoforms in dense core vesicle exocytosis. Am J Physiol Cell Physiol. 2008;294(3):C797–809.
Castle A, Castle D. Ubiquitously expressed secretory carrier membrane proteins (SCAMPs) 1–4 mark different pathways and exhibit limited constitutive trafficking to and from the cell surface. J Cell Sci. 2005;118:3769–80.
Biewenga P, Buist MR, Moerland PD, Ver Loren van Themaat E, van Kampen AH, ten Kate FJ, et al. Gene expression in early stage cervical cancer. Gynecol Oncol. 2008;108:520–6.
Kim JH, Lee JY, Lee KT, Lee KH, Jang KT, Heo JS, et al. RGS16 and FosB underexpressed in pancreatic cancer with lymph node metastasis promote tumor progression. Tumour Biol. 2010;31:541–8.
Kim K, Kim KH, Cho HK, Kim HY, Kim HH, Cheong J. SHP (small heterodimer partner) suppresses the transcriptional activity and nuclear localization of Hedgehog signaling protein Gli1. Biochem J. 2010;427:413–22.
Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–25.
Ziyad S, Iruela-Arispe ML. Molecular mechanisms of tumor angiogenesis. Genes Cancer. 2011;2(12):1085–96.
He HZ, Wang N, Zhang J, Zheng L, Zhang YM. Tas13D inhibits growth of SMMC-7721 cell via suppression VEGF and EGF expression. Asian Pac J Cancer Prev. 2012;13(5):2009–14.
Min BS, Kim NK, Jeong HC, Chung HC. High levels of serum VEGF and TIMP-1 are correlated with colon cancer liver metastasis and intrahepatic recurrence after liver resection. Oncol Lett. 2012;4(1):123–30.
Singleton DR, Wu TT, Castle JD. Three mammalian SCAMPs (secretory carrier membrane proteins) are highly related products of distinct genes having similar subcellular distributions. J Cell Sci. 1997;110:2099–107.
Fernandez-Chacon R, Sudhof TC. Novel SCAMPs lacking NPF repeats: ubiquitous and synaptic vesicle-specific forms implicate SCAMPs in multiple membrane-trafficking functions. J Neurosci. 2000;20:7941–50.
Lam SK, Siu CL, Hillmer S, Jang S, An G, Robinson DG, et al. Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell. 2007;19(1):296–319.
Fernandez-Chacon R, Alvarez de Toledo G, Hammer RE, Sudhof TC. Analysis of SCAMP1 function in secretory vesicle exocytosis by means of gene targeting in mice. J Biol Chem. 1999;274:32551–4.
Sun HC, Qiu ZJ, Liu J, Sun J, Jiang T, Huang KJ, et al. Expression of hypoxia-inducible factor-1 alpha and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosis. Int J Oncol. 2007;30:1359–67.
Wu TT, Castle JD. Tyrosine phosphorylation of selected secretory carrier membrane proteins, SCAMP1 and SCAMP3, and association with the EGF receptor. Mol Biol Cell. 1998;9:1661–74.
Sun P, Yu H, Zhang WQ, Hu M, Lv R. Lentivirus-mediated siRNA targeting VEGF inhibits gastric cancer growth in vivo. Oncol Rep. 2012;28(5):1687–92.
Dobrila-Dintinjana R, Vanis N, Dintinjana M, Radić M. Etiology and oncogenesis of pancreatic carcinoma. Coll Antropol. 2012;36(3):1063–7.
Wang YY, Cui QC. Recent advances in gene change of pancreatic cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26(1):79–82.
Shin SH, Kim SC, Hong SM, Kim YH, Song KB, Park KM, et al. Genetic alterations of K-ras, p53, c-erbB-2, and DPC4 in pancreatic ductal adenocarcinoma and their correlation with patient survival. Pancreas. 2013;42(2):216–22.
Scarpa A, Orlandini S, Moore PS, Lemoine NR, Beghelli S, Baron A, et al. Dpc4 is expressed in virtually all primary and metastatic pancreatic endocrine carcinomas. Virchows Arch. 2002;440(2):155–9.
Schwarte-Waldhoff I, Volpert OV, Bouck NP, Sipos B, Hahn SA, Klein-Scory S, et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci U S A. 2000;97(17):9624–9.
Acknowledgments
This work was supported by the Samsung Biomedical Research Institute grant, # SBRI C-B1-118-2.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Lee, K.T., Lee, J.Y. et al. Inhibition of SCAMP1 suppresses cell migration and invasion in human pancreatic and gallbladder cancer cells . Tumor Biol. 34, 2731–2739 (2013). https://doi.org/10.1007/s13277-013-0825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0825-9