Abstract
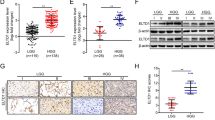
Malignant gliomas display over-expression of the receptor tyrosine kinase EphA2. However, expression levels of the EphA2 ligand, EphrinA1, have not been fully elucidated. Seventy-eight patients with primary gliomas were included in this study who underwent surgical resection, radiation, and chemotherapy. The expression of EphA2 and EphrinA1 in tumors was assessed by immunohistochemistry and was statistically analyzed in combination with the follow-up data of patients. EphA2 was highly expressed in most malignant gliomas, but EphrinA1 was expressed at low levels in these tumors. The increased EphA2 expression is associated with higher-grade histology and poor patient prognosis. Contrary to this, the increased EphrinA1 expression is associated with lower-grade histology, but not associated with poor patient prognosis. Moreover, patients with tumors positive for EphA2 and negative for EphrinA1 had significantly shorter overall and progression-free survival than patients with tumors positive for both EphA2 and EphrinA1, negative for both EphA2 and EphrinA1, or negative for EphA2 and positive for EphrinA1. RNAi-mediated suppression of endogenous EphA2 in human glioblastoma multiforme cells resulted in increased EphrinA1 levels, as well as decreased cell viability, anchorage independence and in vitro invasion, and increased apoptosis. Furthermore, suppression of EphA2 resulted in delayed tumor growth in mice xenografts. Together, these data indicate that up-regulation of EphA2 and down-regulation of Ephrina1 may correlate with poor prognosis for patients with high-grade glioma. EphA2 suppression partially reversed the aggressive phenotypes of malignant gliomas, possibly through up-regulating EphrinA1 expression, which may help explain how EphA2 modulates the malignant progression of gliomas.





Similar content being viewed by others
References
Kleihues P, Cavenee WK. World health organization classification of tumours: pathology and genetics of tumours of the nervous system. Lyon: IARC; 2000.
Lindberg RA, Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990;10:6316–24.
Sulman EP, Tang XX, Allen C, Biegel JA, Pleasure DE, Brodeur GM, et al. ECK, a human EPH-related gene, maps to 1p36.1, a common region of alteration in human cancers. Genomics. 1997;40:371–4.
Kinch MS, Moore MB, Harpole Jr DH. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–8.
Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther. 2004;11:757–66.
Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, et al. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353–60.
Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, et al. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–7.
Kataoka H, Igarashi H, Kanamori M, Ihara M, Wang JD, Wang YJ, et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136–41.
Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, et al. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–80.
Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin Ther Targets. 2005;9:1179–87.
Herrem CJ, Tatsumi T, Olson KS, Shirai K, Finke JH, Bukowski RM, et al. Expression of EphA2 is prognostic of disease-free interval and overall survival in surgically treated patients with renal cell carcinoma. Clin Cancer Res. 2005;11:226–31.
Xu F, Zhong W, Li J, Shanshen Z, Cui J, Nesland JM, et al. Predictive value of EphA2 and EphrinA-1 expression in oesophageal squamous cell carcinoma. Anticancer Res. 2005;25:2943–50.
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–56.
Easty DJ, Hill SP, Hsu MY, Fallowfield ME, Florenes VA, Herlyn M, et al. Up-regulation of ephrin-A1 during melanoma progression. Int J Cancer. 1999;84:494–501.
Holzman LB, Marks RM, Dixit VM. A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol. 1990;10:5830–8.
Carles-Kinch K, Kilpatrick KE, Stewart JC, Kinch MS. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res. 2002;62:2840–7.
Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–30.
Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–38.
Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–6.
Han L, Dong Z, Qiao Y, Kristensen GB, Holm R, Nesland JM, et al. The clinical significance of EphA2 and Ephrin A-1 in epithelial ovarian carcinomas. Gynecol Oncol. 2005;99:278–86.
Wu D, Suo Z, Kristensen GB, Li S, Troen G, Holm R, et al. Prognostic value of EphA2 and EphrinA-1 in squamous cell cervical carcinoma. Gynecol Oncol. 2004;94:312–9.
Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–22.
Li X, Wang Y, Wang Y, Zhen H, Yang H, Fei Z, et al. Expression of EphA2 in human astrocytic tumors: correlation with pathologic grade, proliferation and apoptosis. Tumour Biol. 2007;28:165–72.
Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–23.
Wang LF, Fokas E, Bieker M, Rose F, Rexin P, Zhu Y, et al. Increased expression of EphA2 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. Oncol Rep. 2008;19:151–6.
Zhou Z, Yuan X, Li Z, Tu H, Li D, Qing J, et al. RNA interference targeting EphA2 inhibits proliferation, induces apoptosis, and cooperates with cytotoxic drugs in human glioma cells. Surg Neurol. 2008;70:562–8.
Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–9.
Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat Cell Biol. 2002;4:565–73.
Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Ligation of EphA2 by Ephrin A1-Fc inhibits pancreatic adenocarcinoma cellular invasiveness. Biochem Biophys Res Commun. 2004;320:1096–102.
Liu DP, Wang Y, Koeffler HP, Xie D. Ephrin-A1 is a negative regulator in glioma through down-reguation of EphA2 and FAK. Int J Oncol. 2007;30:865–71.
Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795–806.
Acknowledgments
We thank Dr. Harry C. Wang (Nastech Pharmaceutical Company Inc., USA) for revising the language of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xia Li, Li Wang, and Jian-Wen Gu contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Li, X., Wang, L., Gu, JW. et al. Up-regulation of EphA2 and down-regulation of EphrinA1 are associated with the aggressive phenotype and poor prognosis of malignant glioma. Tumor Biol. 31, 477–488 (2010). https://doi.org/10.1007/s13277-010-0060-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-010-0060-6