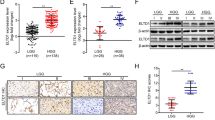
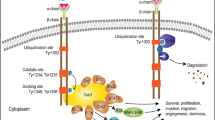
Abstract
Among the gliomas, glioblastoma (GBM) is the highest grade and the most malignant glioma tumor. GATA2 is a hematopoietic factor that has been intensely studied in hematopoietic malignancies. Recently, the functions of GATA2 as an oncogene in other types of human cancer have been reported. However, no role for GATA2 in the development and progression of glioma has been reported to date. In the present study, we found that the expression level of GATA2 is upregulated in GBM and is correlated with GBM outcome. Ectopic expression of GATA2 or RNAi-mediated knockdown of GATA2 significantly enhanced or inhibited proliferation, migration and invasion of glioma cells. Moreover, we found that epidermal growth factor receptor and extracellular signal-regulated kinase, as upstream components of the signaling pathway, upregulate GATA2 expression; moreover, GATA2 promotes Elk-1 expression. Therefore, a genetic approach or pharmacological intervention targeting GATA2 could potentially serve as an effective strategy for treating glioma patients.






Similar content being viewed by others
References
Ahluwalia MS, Chang SM. Medical therapy of gliomas. J Neurooncol. 2014;119:503–12.
Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, Heber-Katz E, Curtis MT, Cotzia P, Hooper DC. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19:3776–86.
Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomark Prev. 2014;23:1985–96.
Martinez R, Schackert G, Yaya-Tur R, Rojas-Marcos I, Herman JG, Esteller M. Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme. J Neurooncol. 2007;83:91–3.
Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, Barker FG 2nd, Aldape K. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–8.
Zheng R, Blobel GA. GATA transcription factors and cancer. Genes Cancer. 2010;1:1178–88.
Bresnick EH, Katsumura KR, Lee HY, Johnson KD, Perkins AS. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 2012;40:5819–31.
Chlon TM, Crispino JD. Combinatorial regulation of tissue specification by GATA and FOG factors. Development. 2012;139:3905–16.
Vicente C, Conchillo A, García-Sánchez MA, Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82:1–17.
Wang Y, He X, Ngeow J, Eng C. GATA2 negatively regulates PTEN by preventing nuclear translocation of androgen receptor and by androgen-independent suppression of PTEN transcription in breast cancer. Hum Mol Genet. 2012;21:569–76.
Kumar MS, Hancock DC, Molina-Arcas M, Steckel M, East P, Diefenbacher M, Armenteros-Monterroso E, Lassailly F, Matthews N, Nye E, Stamp G, Behrens A, Downward J. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell. 2012;149:642–55.
Chiang YT, Wang K, Fazli L, Qi RZ, Gleave ME, Collins CC, Gout PW, Wang Y. GATA2 as a potential metastasis-driving gene in prostate cancer. Oncotarget. 2014;5:451–61.
Kancha RK, von Bubnoff N, Peschel C, Duyster J. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin Cancer Res. 2009;15:460–7.
Liu KJ, Chen CT, Hu WS, Hung YM, Hsu CY, Chuang BF, Juang SH. Expression of cytoplasmic-domain substituted epidermal growth factor receptor inhibits tumorigenicity of EGFR-overexpressed human glioblastoma multiforme. Int J Oncol. 2004;24:581–90.
Carlsson J, Ren ZP, Wester K, Sundberg AL, Heldin NE, Hesselager G, Persson M, Gedda L, Tolmachev V, Lundqvist H, Blomquist E, Nistér M. Planning for intracavitary anti-EGFR radionuclide therapy of gliomas. Literature review and data on EGFR expression. J Neurooncol. 2006;77:33–45.
Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–87.
Mut M, Lule S, Demir O, Kurnaz IA, Vural I. Both mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinases (ERK) 1/2 and phosphatidylinositide-3-OH kinase (PI3 K)/Akt pathways regulate activation of E-twenty-six (ETS)-like transcription factor 1 (Elk-1) in U138 glioblastoma cells. Int J Biochem Cell Biol. 2012;44:302–10.
Wu P, Wee P, Jiang J, Chen X, Wang Z. Differential regulation of transcription factors by location-specific EGF receptor signaling via a spatio-temporal interplay of ERK activation. PLoS ONE. 2012;7:e41354.
Menon LG, Pratt J, Yang HW, Black PM, Sorensen GA, Carroll RS. Imaging of human mesenchymal stromal cells: homing to human brain tumors. J Neurooncol. 2012;107:257–67.
Terasaki M, Sugita Y, Arakawa F, Okada Y, Ohshima K, Shigemori M. CXCL12/CXCR4 signaling in malignant brain tumors: a potential pharmacological therapeutic target. Brain Tumor Pathol. 2011;28:89–97.
Broniscer A, Baker SJ, Stewart CF, Merchant TE, Laningham FH, Schaiquevich P, Kocak M, Morris EB, Endersby R, Ellison DW, Gajjar A. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin Cancer Res. 2009;15:701–7.
Ménard S, Casalini P, Campiglio M, Pupa SM, Tagliabue E. Role of HER2/neu in tumor progression and therapy. Cell Mol Life Sci. 2004;61:2965–78.
Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J Cell Physiol. 2002;192:151–9.
Labussière M, Boisselier B, Mokhtari K, Di Stefano AL, Rahimian A, Rossetto M, Ciccarino P, Saulnier O, Paterra R, Marie Y, Finocchiaro G, Sanson M. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83:1200–6.
Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr Cancer Drug Targets. 2012;12:197–209.
Moriguchi T, Yamamoto M. A regulatory network governing Gata1 and Gata2 gene transcription orchestrates erythroid lineage differentiation. Int J Hematol. 2014;100:417–24.
Goncharenko-Khaider N, Matte I, Lane D, Rancourt C, Piché A. Ovarian cancer ascites increase Mcl-1 expression in tumor cells through ERK1/2-Elk-1 signaling to attenuate TRAIL-induced apoptosis. Mol Cancer. 2012;11:84.
Acknowledgments
This work was supported by University Graduate Students’ Scientific Research Innovative Program of Jiangsu Province, China (CXLX12-0843); National Natural Science Foundation of China (81172400, 81272799 and 81472739); Scientific and Technological Development Program of Suzhou, China (SYS201477, SYSD2012090); and Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhongyong Wang, Hui Yuan and Chao Sun have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Z., Yuan, H., Sun, C. et al. GATA2 promotes glioma progression through EGFR/ERK/Elk-1 pathway. Med Oncol 32, 87 (2015). https://doi.org/10.1007/s12032-015-0522-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0522-1