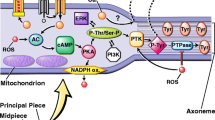
Abstract
Oxidative stress caused by the production of reactive oxygen species (ROS) in excess of the defense capability of the body’s antioxidative system is one of the primary reasons of sperm dysfunction. ROS, including free radicals, oxygen ions, and peroxides, are by-products of normal cellular metabolism. These molecules are important in physiological processes and cellular signaling pathways. Moderate amounts of ROS are indispensable for sperm physiology, such as capacitation, hyperactivation, and sperm-egg fusion. However, relatively high level of ROS can disrupt the integrity of sperm DNA and limit the fertilizing potential because of the compromised lipids and proteins in the sperm membrane. The antioxidative system of semen is composed of enzymatic and non-enzymatic antioxidants which interact with each other to maintain an intricate balance between ROS production and scavenging system. This review highlights the mechanisms of ROS production and elimination as well as the physiological and pathological effects on spermatozoa.
Similar content being viewed by others
References
Trussell, J. C. Optimal diagnosis and medical treatment of male infertility. Semin Reprod Med 31:235–236 (2013).
McLachlan, R. I. & de Kretser, D. M. Male infertility: the case for continued research. Med J Aust 174:116–117 (2001).
Aitken, R. J., Smith, T. B., Jobling, M. S., Baker, M. A. & De Iuliis, G. N. Oxidative stress and male reproductive health. Asian J Androl 16:31–38 (2014).
Da Ros, V. G. et al. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev Biol 320:12–18 (2008).
MacLeod, J. The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol 138:512–518 (1943).
Greco, E. et al. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl 26:349–353 (2005).
Aitken, R. J., Baker, M. A., De Iuliis, G. N. & Nixon, B. New insights into sperm physiology and pathology. Handb Exp Pharmacol 198:99–115 (2010).
Butler, A. et al. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am J Pathol 161:1061–1075 (2002).
Bansal, A. K. & Bilaspuri, G. S. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int (2010), doi: 10.4061/2011/686137
Tremellen, K. Oxidative stress and male infertility -a clinical perspective. Hum Reprod Update 14:243–258 (2008).
Cheeseman, K. H. & Slater, T. F. An introduction to free radical biochemistry. Br Med Bull 49:481–493 (1993).
Henkel, R. R. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. J Asian J Androl 13:43–52 (2011).
Bae, Y. S., Oh, H., Rhee, S. G. & Yoo, Y. D. Regulation of reactive oxygen species generation in cell signaling. Mol Cells 32:491–509 (2011).
Wood, Z. A., Poole, L. B. & Karplus, P. A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300:650–653 (2003).
Paulsen, C. E. & Carroll, K. S. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev 113:4633–4679 (2013).
Bindoli, A., Fukuto, J. M. & Forman, H. J. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal 10:1549–1564 (2008).
St-Pierre, J., Buckingham, J. A., Roebuck, S. J. & Brand, M. D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277:44784–44790 (2002).
Panieri, E. & Santoro, M. M. ROS signaling and redox biology in endothelial cells. Cell Mol Life Sci 72:3281–3303 (2015).
Forman, H. J., Davies, K. J. A. & Ursini, F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med 66:24–35 (2014).
Sagi, O., Wolfson, M., Utko, N., Muradian, K. & Fraifeld, V. p66 ShcA and ageing: modulation by longevitypromoting agent aurintricarboxylic acid. Mech Ageing Dev 126:249–254 (2005).
Trinei, M. et al. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidationdamaged DNA and oxidative stress-induced apoptosis. Oncogene 21:3872–3878 (2002).
Giorgio, M. et al. Electron transfer between cytochrome c and p66 Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233 (2005).
Paulsen, C. E. & Carroll, K. S. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol 5:47–62 (2009).
Chen, K., Craige, S. E. & Keaney, J. F. Jr. Downstream targets and intracellular compartmentalization in Nox signaling. Antioxid Redox Signal 11:2467–2480 (2009).
Ameziane-El-Hassani, R. et al. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 34:30046–30054 (2005).
Garrido, N., Meseguer, M., Simon, C., Pellicer, A. & Remohi, J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J Androl 6:59–66 (2004).
Aitken, R. J. The Amoroso Lecture The human spermatozoon-a cell in crisis?. J Reprod Fertil 115:1–7 (1999).
Agarwal, A. & Sekhon, L. H. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil (Camb) 13:217–225 (2010).
Plante, M., De Lamirande, E. & Gagnon, C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil Steril 62:387–393 (1994).
Kaleli, S., Öçer, F., Irez, T., Budak, E. & Aksu, M. F. Does leukocytospermia associate with poor semen parameters and sperm functions in male infertility?: The role of different seminal leukocyte concentrations. Eur J Obstet Gynecol Reprod Biol 89:185–191 (2000).
Aziz, N., Agarwal, A., Lewis-Jones, I., Sharma, R. K. & Thomas, A. J. Jr. Novel associations between specific sperm morphological defects and leukocytospermia. Fertil Steril 82:621–627 (2004).
Tremellen, K. & Tunc, O. Macrophage activity in semen is significantly correlated with sperm quality in infertile men. Int J Androl 33:823–831 (2010).
Mupfiga, C., Fisher, D., Kruger, T. & Henkel, R. The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst Biol Reprod Med 59:304–311 (2013).
Morgan, M. J. & Liu, Z. G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115 (2011).
Tremellen, K. & Tuncx, K. Macrophage activity in semen is significantly correlated with sperm quality in infertile men. Int J Androl 33:823–831 (2010).
Barraud-Lange, V. et al. Seminal leukocytes are Good Samaritans for spermatozoa. Fertil Steril 96:1315–1319 (2011).
Kiessling, A. A., Lamparelli, N., Yin, H. Z., Seibel, M. M. & Eyre, R. C. Semen leukocytes: friends or foes?. Fertil Steril 64:196–198 (1995).
Tomlinson, M. J., White, A., Barratt, C. L., Bolton, A. E. & Cooke, I. D. The removal of morphologically abnormal sperm forms by phagocytes: a positive role for seminal leukocytes. Hum Reprod 7:517–522 (1992).
Piasecka, M. et al. Novel morphological findings of human sperm removal by leukocytes in in vivo and in vitro conditions: preliminary study. Am J Reprod Immunol 72:348–358 (2014).
Kothari, S., Thompson, A., Agarwal, A. & du Plessis, S. S. Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol 48:425–435 (2010).
Aitken, R. J., Ryan, A. L., Baker, M. A. & McLaughlin, E. A. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radic Biol Med 36:994–1010 (2004).
O’Flaherty, C. & de Souza, A. R. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol Reprod 84:238–247 (2011).
Gil-Guzman, E. et al. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum Reprod 16:1922–1930 (2001).
Fisher, A. B., Dodia, C., Manevich, Y., Chen, J. W. & Feinstein, S. I. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem 30:21326–21334 (1999).
Noblanc, A. et al. Epididymis response partly compensates for spermatozoa oxidative defects in snGPx4 and GPx5 double mutant mice. PLoS One 7:e38565 (2012).
Puglisi, R. et al. The nuclear form of glutathione peroxidase 4 is associated with sperm nuclear matrix and is required for proper paternal chromatin decondensation at fertilization. J Cell Physiol 227:1420–1427 (2012).
Noblanc, A. et al. Epididymis response partly compensates for spermatozoa oxidative defects in snGPx4 and GPx5 double mutant mice. PLoS One 7:e38565 (2012).
Ozkosem, B., Feinstein, S. I., Fisher, A. B. & O’Flaherty, C. Absence of Peroxiredoxin 6 Amplifies the Effect of Oxidant Stress on Mobility and SCSA/CMA3 Defined Chromatin Quality and Impairs Fertilizing Ability of Mouse Spermatozoa. Biol Reprod 94:68 (2016).
Calvin, H. & Bedford, J. Formation of disulphide bonds in the nucleus and accessory structures of mammalian spermatozoa during maturation in the epididymis. J Reprod Fertil Suppl 13:Suppl 13:65–75 (1971).
Fujii, J. & Imai, H. Redox reactions in mammalian spermatogenesis and the potential targets of reactive oxygen species under oxidative stress. Spermatogenesis 4:e979108 (2014).
Awda, B. J. & Buhr, M. M. Extracellular signal-regulated kinases (ERKs) pathway and reactive oxygen species regulate tyrosine phosphorylation in capacitating boar spermatozoa. Biol Reprod 83:750–758 (2010).
Ickowicz, D., Finkelstein, M. & Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl 14:816–821 (2012).
Visconti, P. E. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A 106:667–668 (2009).
Baker, M. A. & Aitken, R. J. The importance of redox regulated pathways in sperm cell biology. Mol Cell Endocrinol 216:47–54 (2004).
Nolan, M. A. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A 101:13483–13488 (2004).
Carlson, A. E., Hille, B. & Babcock, D. F. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol 312:183–192 (2007).
Aitken, R. J., Harkiss, D., Knox, W., Paterson, M. & Irvine, D. S. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci 111:645–656 (1998).
Belén Herrero, M., Chatterjee, S., Lefièvre, L., de Lamirande, E. & Gagnon, C. Nitric oxide interacts with the cAMP pathway to modulate capacitation of human spermatozoa. Free Radic Biol Med 29:522–536 (2000).
de Lamirande, E. & O’Flaherty, C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta 1784:106–115 (2008).
Agarwal, A., Virk, G., Ong, C. & du Plessis, S. S. Effect of oxidative stress on male reproduction. World J Mens Health 32:1–17 (2014).
Lamirande, E. D. & Gagnon, C. Reactive oxygen species and human spermatozoa. J Androl 13:368–378 (1992).
O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J Androl 17:583–590 (2015).
Florman, H. M., Jungnickel, M. K. & Sutton, K. A. Regulating the acrosome reaction. Int J Dev Biol 52:503–510 (2008).
Aitken, R. J. & Clarkson, J. S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 81:459–469 (1987).
Breitbart, H. & Naor, Z. Protein kinases in mammalian sperm capacitation and the acrosome reaction. Rev Reprod 4:151–159 (1999).
Aitken, R. J., Paterson, M., Fisher, H., Buckingham, D. W. & van Duin, M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108:2017–2025 (1995).
Griveau, J. E., Renard, P. & Le Lannou, D. An in vitro promoting role for hydrogen peroxide in human sperm capacitation. Int J Androl 17:300–307 (1997).
Lamirande, E. D. & Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl 16:21–25 (1993).
Goldman, R., Ferber, E. & Zort, U. Reactive oxygen species are involved in the activation of cellular phospholipase A2. FEBS Lett 309:190–192 (1993).
Breitbart, H. & Finkelstein, M. Regulation of Sperm Capacitation and the Acrosome Reaction by PIP 2 and Actin Modulation. Asian J Androl 17:597–600 (2015).
Griveau, J. F., Renard, P. & Lannou, D. Superoxide anion production by human spermatozoa as a part of the ionophore-induced acrosome reaction process. Int J Androl 18:67–74 (1995).
Khosrowbeygi, A. & Zarghami, N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot Essent Fatty Acids 77:117–121 (2007).
Aksoy, Y., Aksoy, H., Altınkaynak, K., Aydin, H. R. & Ozkan, A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot Essent Fatty Acids 75:75–79 (2006).
Bell, M., Wang, R., Hellstrom, W. J. & Sikka, S. C. Effect of cryoprotective additives and cryopreservation protocol on sperm membrane lipid peroxidation and recovery of motile human sperm. J Androl 14:472–478 (1993).
Saalu, L. C. The incriminating role of reactive oxygen species in idiopathic male infertility: an evidence based evaluation. Pak J Biol Sci 13:413–422 (2010).
Kuiper, H. C., Miranda, C. L, Sowell, J. D. & Stevens, J. F. Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J Biol Chem 283:17131–17138 (2008).
Berlett, B. S. & Stadtman, E. R. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316 (1997).
Garrison, W. M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem Rev 87:381–398 (1987).
Levine, R. L., Mosoni, L., Berlett, B. S. & Stadtman, E. R. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A 93:15036–15040 (1996).
Dalle-Donne, I., Rossi, R., Giustarini, D., Milzani, A. & Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38 (2003).
Dean, R. T., Fu, S., Stocker, R. & Davies, M. J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 324:1–18 (1997).
Agarwal, A. & Said, T. M. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int 95:503–507 (2005).
Goldberg, R. B., Geremia, R. & Bruce, W. R. Histone synthesis and replacement during spermatogenesis in the mouse. Differentiation 7:167–180 (1977).
Saowaros, W. & Panyim, S. The formation of disulfide bonds in human protamines during sperm maturation. Experientia 35:191–192 (1979).
Sakkas, D. & Alvarez, J. G. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 93:1027–1036 (2010).
Ramos, L. et al. Incomplete nuclear transformation of human spermatozoa in oligo-astheno-teratospermia: characterization by indirect immunofluorescence of chromatin and thiol status. Hum Reprod 23:259–270 (2008).
Moustafa, M. H. et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod 19:129–138 (2004).
Barroso, G., Morshedi, M. & Oehninger, S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod 15:1338–1344 (2000).
Saleh, R. A. et al. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril 80:1431–1436 (2003).
Burns, P. D. & Herickhoff, L. A. DNA Fragmentation as an Early Indicator of Extender Efficacy: A Preliminary Study. J Equine Vet Sci 34:375–379 (2014).
Kumar, A., Pottiboyina, V. & Sevilla, M. D. Hydroxyl radical (OH•) reaction with guanine in an aqueous environment: a DFT study. J Phys Chem B 115:15129–15137 (2011).
Madugundu, G. S., Cadet, J. & Wagner, J. R. Hydroxylradical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic Acids Research 42:7450–7460 (2014).
Valko, M., Izakovic, M., Mazur, M., Rhodes, C. J. & Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56 (2004).
Pogozelski, W. K. & Tullius, T. D. Oxidative strand scission of nucleic acids: routes initiated by hydrogen abstraction from the sugar moiety. Chem Rev 98:1089–1108 (1998).
Steenken, S. Purine bases, nucleosides, and nucleotides: aqueous solution redox chemistry and transformation reactions of their radical cations and e-and OH adducts. Chem Rev 89:503–520 (1989).
Burrows, C. J. & Muller, J. G. Oxidative nucleobase modifications leading to strand scission. Chem Rev 98:1109–1152 (1998).
Wu, C., Chen, S. T., Peng, K. H., Cheng, T. J. & Wu, K. Y. Concurrent quantification of multiple biomarkers indicative of oxidative stress status using liquid chromatography-tandem mass spectrometry. Anal Biochem (2016), doi: 10.1016/j.ab.2016.07.030
Makker, K., Agarwal, A. & Sharma, R. Oxidative stress & male infertility. Indian J Med Res 129:357–367 (2009).
Gaur, D. S., Talekar, M. & Pathak, V. P. Effect of cigarette smoking on semen quality of infertile men. Singapore Med J 48:119–123 (2007).
Ramlau-Hansen, C. H. et al. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum Reprod 22:188–196 (2007).
Hammadeh, M. E., Hamad, M. F., Montenarh, M. & Fischer-Hammadeh, C. Protamine contents and P1/ P2 ratio in human spermatozoa from smokers and non-smokers. Hum Reprod 25:2708–2720 (2010).
Saleh, R. A., Agarwal, A., Sharma, R. K., Nelson, D. R. & Thomas, A. J. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril 78:491–499 (2002).
Pryor, W. A. & Stone, K. Oxidants in cigarette smoke radicals, hydrogen peroxide, peroxynitrate, and peroxynitritea. Ann N Y Acad Sci 686:12–27 (1993).
Zhu, Q. et al. Ethanol exposure enhances apoptosis within the testes. Alcohol Clin Exp Res 24:1550–1556 (2000).
Maneesh, M., Dutta, S., Chakrabarti, A. & Vasudevan, D. M. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol 50:291–296 (2006).
Kim, J. S. et al. Genistein Mitigates Radiation-induced Testicular Injury. Phytother Res 26:1119–1125 (2012).
Angelopoulou, R., Lavranos, G. & Manolakou, P. ROS in the aging male: model diseases with ROSrelated pathophysiology. Reprod Toxicol 28:167–171 (2009).
Coccuzza, M., Sikka, S. C., Athayde, K. S. & Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol 33:603–621 (2007).
Esmekaya, M. A., Ozer, C. & Seyhan, N. 900 MHz pulse-modulated radiofrequency radiation induces oxidative stress on heart, lung, testis and liver tissues. Gen Physiol Biophys 30:84–89 (2011).
Friedman, J., Kraus, S., Hauptman, Y., Schiff, Y. & Seger, R. Mechanism of shortterm ERK activation by electromagnetic fields at mobile phone frequencies. Biochem J 405:559–568 (2007).
Agarwal, A. et al. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil Steril 92:1318–1325 (2008).
Pino, J. A. et al. Differential effects of temperature on reactive oxygen/nitrogen species production in rat pachytene spermatocytes and round spermatids. Reproduction 145:203–212 (2013).
Esfandiari, N. et al. Effects of temperature on sperm motion characteristics and reactive oxygen species. Int J Fertil Womens Med 47:227–235 (2002).
Wang, A. W. et al. Reactive oxygen species generation by seminal cells during cryopreservation. Urology 49:921–925 (1997).
Adedara, I. A. & Farombi, E. O. Induction of oxidative damage in the testes and spermatozoa and hematotoxicity in rats exposed to multiple doses of ethylene glycol monoethyl ether. Hum Exp Toxicol 29:801–812 (2010).
Kazemi, S., Feizi, F., Aghapour, F., Joorsaraee, G. A. & Moghadamnia, A. A. Histopathology and histomorphometric investigation of bisphenol a and nonylphenol on the male rat reproductive system. N Am J Med Sci 8:215–221 (2016).
Hong, J. et al. Exposure of preimplantation embryos to low-dose bisphenol A impairs testes development and suppresses histone acetylation of StAR promoter to reduce production of testosterone in mice. Mol Cell Endocrinol 427:101–111 (2016).
Chitra, K. C., Latchoumycandane, C. & Mathur, P. P. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology 185:119–127 (2003).
Kazemi, S. et al. Induction Effect of Bisphenol A on Gene Expression Involving Hepatic Oxidative Stress in Rat. Oxid Med Cell Longev 2016:6298515 (2016).
Lee, E. et al. Effect of di(n-butyl) phthalate on testicular oxidative damage and antioxidant enzymes in hyperthyroid rats. Environ Toxicol 22:245–255 (2007).
Kasahara, E. et al. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J 365:849–856 (2002).
Walczak-Jedrzejowska, R., Wolski, J. K. & Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol 6:60–67 (2013).
Aitken, R. J. & Roman, S. D. Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol 636:154–171 (2008).
Chen, S. J., Allam, J. P., Duan, Y. G. & Haidl, G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet 288:191–199 (2013).
Fraczek, M. & Kurpisz, M. The redox system in human semen and peroxidative damage of spermatozoa. Postepy Hig Med Dosw (Online) 59:523–534 (2004).
Gałecka, E., Jacewicz, R., Mrowicka, M., Florkowski, A. & Gałecki, P. Antioxidative enzymes-structure, properties, functions. Pol Merkur Lekarski 25:266 (2008).
Lundwall, Å., Bjartell, A. & Olsson, A. Y. Semenogelin I and II, the predominant human seminal plasma proteins, are also expressed in non-genital tissues. Mol Hum Reprod 8:805–810 (2002).
Yeung, C. H. et al. Studies on the origin of redox enzymes in seminal plasma and their relationship with results of in-vitro fertilization. Mol Hum Reprod 4:835–839 (1998).
Vaisberg, C. N., Jelezarsky, L. V., Dishlianova, B. & Chaushev, T. A. Activity, substrate detection and immunolocalization of glutathione peroxidase (GPx) in bovine reproductive organs and semen. Theriogenology 64:416–428 (2005).
Williams, A. C. & Ford, W. C. L. Functional significance of the pentose phosphate pathway and glutathione reductase in the antioxidant defenses of human sperm. Biol Reprod 71:1309–1316 (2004).
Giannattasio, A. et al. Glutathione peroxidase (GPX) activity in seminal plasma of healthy and infertile males. J Endocrinol Invest 25:983–986 (2002).
Atig, F. et al. Altered antioxidant status and increased lipid per-oxidation in seminal plasma of tunisian infertile men. Int J Biol Sci 8:139–149 (2012).
Keskes-Ammar, L. et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl 49:83–94 (2003).
Comhaire, F. H. et al. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins Leukot Essent Fatty Acids 63:159–165 (2000).
Moslemi, M. K. & Tavanbakhsh, S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med 4:99–104 (2011).
Akmal, M. et al. Improvement in human semen quality after oral supplementation of vitamin C. J Med Food 9:440–442 (2006).
Sönmez, M., Türk, G. & Yüce, A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 63:2063–2072 (2005).
Song, G. J., Norkus, E. P. & Lewis, V. Relationship between seminal ascorbic acid and sperm DNA integrity in infertile men. Int J Androl 29:569–575 (2006).
Hogarth, C. A. & Griswold, M. D. The key role of vitamin A in spermatogenesis. J Clin Invest 120:956–962 (2010).
Matthews, R. G. Methylenetetrahydrofolate reductase: a common human polymorphism and its biochemical implications. J Chem Rec 2:4–12 (2002).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr Biol 24:453–462 (2014).
Almbro, M., Dowling, D. K. & Simmons, L. W. Effects of vitamin E and beta-carotene on sperm competitiveness. Ecol Lett 14:891–895 (2011).
Mora-Esteves, C. & Shin, D. Nutrient supplementation: improving male fertility fourfold. Semin Reprod Med 31:293–300 (2013).
Prochaska, H. J. & Talalay, P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res 48:4776–4782 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, S., Li, C., Chen, L. et al. Actions and mechanisms of reactive oxygen species and antioxidative system in semen. Mol. Cell. Toxicol. 13, 143–154 (2017). https://doi.org/10.1007/s13273-017-0015-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-017-0015-8