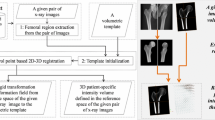
Abstract
We improved the geometrical modeling procedure for fast and accurate reconstruction of orthopedic structures. This procedure consists of medical image segmentation, three-dimensional geometrical reconstruction, and assignment of material properties. The patient-specific orthopedic structures reconstructed by this improved procedure can be used in the virtual surgical planning, 3D printing of real orthopedic structures and finite element analysis. A conventional modeling consists of: image segmentation, geometrical reconstruction, mesh generation, and assignment of material properties. The present study modified the conventional method to enhance software operating procedures. Patient’s CT images of different bones were acquired and subsequently reconstructed to give models. The reconstruction procedures were three-dimensional image segmentation, modification of the edge length and quantity of meshes, and the assignment of material properties according to the intensity of gravy value. We compared the performance of our procedures to the conventional procedures modeling in terms of software operating time, success rate and mesh quality. Our proposed framework has the following improvements in the geometrical modeling: (1) processing time: (femur: 87.16 ± 5.90 %; pelvis: 80.16 ± 7.67 %; thoracic vertebra: 17.81 ± 4.36 %; P < 0.05); (2) least volume reduction (femur: 0.26 ± 0.06 %; pelvis: 0.70 ± 0.47, thoracic vertebra: 3.70 ± 1.75 %; P < 0.01) and (3) mesh quality in terms of aspect ratio (femur: 8.00 ± 7.38 %; pelvis: 17.70 ± 9.82 %; thoracic vertebra: 13.93 ± 9.79 %; P < 0.05) and maximum angle (femur: 4.90 ± 5.28 %; pelvis: 17.20 ± 19.29 %; thoracic vertebra: 3.86 ± 3.82 %; P < 0.05). Our proposed patient-specific geometrical modeling requires less operating time and workload, but the orthopedic structures were generated at a higher rate of success as compared with the conventional method. It is expected to benefit the surgical planning of orthopedic structures with less operating time and high accuracy of modeling.








Similar content being viewed by others
References
Okazaki Y (2012) Development trends of custom-made orthopedic implants. J Artif Organs 15(1):20–25. doi:10.1007/s10047-011-0584-6
Akins R, Abdelgawad AA, Kanlic EM (2012) Computer navigation in orthopedic trauma: safer surgeries with less irradiation and more precision. J Surg Orthop Adv 21(4):187–197
Testori T, Robiony M, Parenti A, Luongo G, Rosenfeld AL, Ganz SD, Mandelaris GA, Del Fabbro M (2014) Evaluation of accuracy and precision of a new guided surgery system: a multicenter clinical study. Int J Periodontics Restor Dent 34(3):59–69. doi:10.11607/prd.1279
Taddei F, Cristofolini L, Martelli S, Gill HS, Viceconti M (2006) Subject-specific finite element models of long bones: an in vitro evaluation of the overall accuracy. J Biomech 39(13):2457–2467. doi:10.1016/j.jbiomech.2005.07.018
Yavari SA, van der Stok J, Ahmadi SM, Wauthle R, Schrooten J, Weinans H, Zadpoor AA (2014) Mechanical analysis of a rodent segmental bone defect model: the effects of internal fixation and implant stiffness on load transfer. J Biomech 47(11):2700–2708. doi:10.1016/j.jbiomech.2014.05.006
Piccinini M, Cugnoni J, Botsis J, Zacchetti G, Ammann P, Wiskott A (2014) Factors affecting subject-specific finite element models of implant-fitted rat bone specimens: critical analysis of a technical protocol. Comput Methods Biomech Biomed Eng 17(13):1403–1417. doi:10.1080/10255842.2012.736502
Ike H, Inaba Y, Kobayashi N, Hirata Y, Yukizawa Y, Aoki C, Choe H, Saito T (2014) Comparison between mechanical stress and bone mineral density in the femur after total hip arthroplasty by using subject-specific finite element analyses. Comput Methods Biomech Biomed Eng. doi:10.1080/10255842.2013.869320
Miechowicz S, Ciszewski A, Janiszewski M, Jamka J, Libura M, Konieczny P (2011) Methods of rapid prototyping in preoperative planning in musculoskeletal reconstructive surgery. Chir Narzadow Ruchu Ortop Pol 76(2):63–68
Tam MD, Latham T, Brown JR, Jakeways M (2014) Use of a 3D Printed hollow aortic model to assist EVAR planning in a case with complex neck anatomy: potential of 3D printing to improve patient outcome. J Endovasc Ther 21(5):760–762. doi:10.1583/14-4810l.1
Poelert S, Valstar E, Weinans H, Zadpoor AA (2013) Patient-specific finite element modeling of bones. Proc Inst Mech Eng [H] 227(4):464–478. doi:10.1177/0954411912467884
Sprouse C, DeMenthon D, Gammie J, Burlina P (2011) Patient-specific modeling of stress/strain for surgical planning and guidance. In Conference Proceedings : annual international conference of the IEEE engineering in medicine and biology, pp 4309–4313. doi:10.1109/iembs.2011.6091070
Lee JH, Baek MH, Kim YE, Seo JH, Song DR, Ryu HS, Lee CK, Chang BS (2013) Finite element modeling of stress distribution in intervertebral spacers of different surface geometries. Artif Organs 37(11):1014–1020. doi:10.1111/aor.12107
Pankaj P (2013) Patient-specific modelling of bone and bone-implant systems: the challenges. Int J Numer Methods Biomed Eng 29(2):233–249. doi:10.1002/cnm.2536
Burkhart TA, Andrews DM, Dunning CE (2013) Finite element modeling mesh quality, energy balance and validation methods: a review with recommendations associated with the modeling of bone tissue. J Biomech 46(9):1477–1488. doi:10.1016/j.jbiomech.2013.03.022
Kim CH, Zhang H, Mikhail G, von Stechow D, Muller R, Kim HS, Guo XE (2007) Effects of thresholding techniques on microCT-based finite element models of trabecular bone. J Biomech Eng 129(4):481–486. doi:10.1115/1.2746368
Blanchard R, Dejaco A, Bongaers E, Hellmich C (2013) Intravoxel bone micromechanics for microCT-based finite element simulations. J Biomech 46(15):2710–2721. doi:10.1016/j.jbiomech.2013.06.036
Ji S, Ford JC, Greenwald RM, Beckwith JG, Paulsen KD, Flashman LA, McAllister TW (2011) Automated subject-specific, hexahedral mesh generation via image registration. Finite Elem Anal Des 47(10):1178–1185. doi:10.1016/j.finel.2011.05.007
Huang Y, Su Y, Rorden C, Dmochowski J, Datta A, Parra LC (2012) An automated method for high-definition transcranial direct current stimulation modeling. In Conference Proceedings: annual international conference of the IEEE engineering in medicine and biology society, pp 5376–5379. doi:10.1109/embc.2012.6347209
Lievers WB, Kent RW (2013) Patient-specific modelling of the foot: automated hexahedral meshing of the bones. Comput Methods Biomech Biomed Eng 16(12):1287–1297. doi:10.1080/10255842.2012.668538
Suero EM, Hufner T, Stubig T, Krettek C, Citak M (2010) Use of a virtual 3D software for planning of tibial plateau fracture reconstruction. Injury 41(6):589–591. doi:10.1016/j.injury.2009.10.053
Shim VB, Battley M, Anderson IA, Munro JT (2014) Validation of an efficient method of assigning material properties in finite element analysis of pelvic bone. Comput Methods Biomech Biomed Eng. doi:10.1080/10255842.2014.920831
Reed DA, Porro LB, Iriarte-Diaz J, Lemberg JB, Holliday CM, Anapol F, Ross CF (2011) The impact of bone and suture material properties on mandibular function in Alligator mississippiensis: testing theoretical phenotypes with finite element analysis. J Anat 218(1):59–74. doi:10.1111/j.1469-7580.2010.01319.x
Chen G, Schmutz B, Epari D, Rathnayaka K, Ibrahim S, Schuetz MA, Pearcy MJ (2010) A new approach for assigning bone material properties from CT images into finite element models. J Biomech 43(5):1011–1015. doi:10.1016/j.jbiomech.2009.10.040
Xin P, Nie P, Jiang B, Deng S, Hu G, Shen SG (2013) Material assignment in finite element modeling: heterogeneous properties of the mandibular bone. J Craniofac Surg 24(2):405–410. doi:10.1097/SCS.0b013e31827ff137
Zhang GD, Liao WJ, Tao SX, Mao WY, Chen JQ, Zheng XH, FH Z (2009) Methods for material assignment of finite element analysis with femurs. J Clin Rehabil Tissue Eng Res 13(43):8436–8441. doi:10.3969/j.issn.1673-8225.2009.52.012
Wang SJ (2004) Determination of orthotropic bone elastic constants using FEA and modal analysis by Taylor WR et al. [J Biomech. (2002) Vol. 35, pp. 767-773]. Journal of biomechanics 37(3):421
Rho JY, Hobatho MC, Ashman RB (1995) Relations of mechanical properties to density and CT numbers in human bone. Med Eng Phys 17(5):347–355
Oda S, Katahira K, Utsunomiya D, Takaoka H, Honda K, Noda K, Oshima S, Yuki H, Namimoto T, Yamashita Y (2014) Improved image quality at 256-slice coronary CT angiography in patients with a high heart rate and coronary artery disease: comparison with 64-slice CT imaging. Acta Radiol. doi:10.1177/0284185114555152
Gulliksrud K, Stokke C, Martinsen AC (2014) How to measure CT image quality: variations in CT-numbers, uniformity and low contrast resolution for a CT quality assurance phantom. Phys Medica 30(4):521–526. doi:10.1016/j.ejmp.2014.01.006
Shen G, Deng H, Hu S, Jia Z (2014) Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skelet Radiol 43(11):1503–1513. doi:10.1007/s00256-014-1903-9
Lim JH, Jun BC, Song SW (2013) Clinical feasibility of multiplanar reconstruction images of temporal Bone CT in the diagnosis of temporal bone fracture with otic-capsule-sparing facial nerve paralysis. Indian J Otolaryngol Head Neck Surg 65(3):219–224. doi:10.1007/s12070-011-0471-8
Razi T, Niknami M, Alavi Ghazani F (2014) Relationship between Hounsfield Unit in CT Scan and Gray Scale in CBCT. J Dent Res Dent Clin Dent Prospect 8(2):107–110. doi:10.5681/joddd.2014.019
Sutradhar A, Park J, Carrau D, Miller MJ (2014) Experimental validation of 3D printed patient-specific implants using digital image correlation and finite element analysis. Comput Biol Med 52:8–17. doi:10.1016/j.compbiomed.2014.06.002
Kang H, Long JP, Urbiel Goldner GD, Goldstein SA, Hollister SJ (2012) A paradigm for the development and evaluation of novel implant topologies for bone fixation: implant design and fabrication. J Biomech 45(13):2241–2247. doi:10.1016/j.jbiomech.2012.06.011
Acknowledgments
This work is supported in part by the 863 Program of China under Grant 2012AA02A603, in part by the Guangzhou science and technology planning project under Grant 2014J4100153, in part by the Science and technology projects in Guangdong Province under Grant 2014B090901055, and in part by the Science and technology projects in Guangdong Province under Grant 2014A020212176.
Author information
Authors and Affiliations
Corresponding author
Appendix
Rights and permissions
About this article
Cite this article
Huang, H., Xiang, C., Zeng, C. et al. Patient-specific geometrical modeling of orthopedic structures with high efficiency and accuracy for finite element modeling and 3D printing. Australas Phys Eng Sci Med 38, 743–753 (2015). https://doi.org/10.1007/s13246-015-0402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-015-0402-1