Abstract
Background
Primary or metastatic osseous and soft tissue lesions can be treated by ablation techniques.
Methods
These techniques are classified into chemical ablation (including ethanol or acetic acid injection) and thermal ablation (including laser, radiofrequency, microwave, cryoablation, radiofrequency ionisation and MR-guided HIFU). Ablation can be performed either alone or in combination with surgical or other percutaneous techniques.
Results
In most cases, ablation provides curative treatment for benign lesions and malignant lesions up to 3 cm. Furthermore, it can be a palliative treatment providing pain reduction and local control of the disease, diminishing the tumour burden and mass effect on organs. Ablation may result in bone weakening; therefore, whenever stabilisation is undermined, bone augmentation should follow ablation depending on the lesion size and location.
Conclusion
Thermal ablation of bone and soft tissues demonstrates high success and relatively low complication rates. However, the most common complication is the iatrogenic thermal damage of surrounding sensitive structures. Nervous structures are very sensitive to extremely high and low temperatures with resultant transient or permanent neurological damage. Thermal damage can cause normal bone osteonecrosis in the lesion’s periphery, surrounding muscular atrophy and scarring, and skin burns. Successful thermal ablation requires a sufficient ablation volume and thermal protection of the surrounding vulnerable structures.
Teaching points
• Percutaneous ablations constitute a safe and efficacious therapy for treatment of osteoid osteoma.
• Ablation techniques can treat painful malignant MSK lesions and provide local tumour control.
• Thermal ablation of bone and soft tissues demonstrates high success and low complication rates.
• Nerves, cartilage and skin are sensitive to extremely high and low temperatures.
• Successful thermal ablation occasionally requires thermal protection of the surrounding structures.
Similar content being viewed by others
Introduction
During the last decades, technological evolution in the fields of both imaging and instrumentation has led to the development of minimally invasive percutaneous ablative techniques for the treatment of benign and malignant bone tumours. Nowadays, percutaneous ablation techniques in the musculoskeletal system include chemical ablation (i.e. injection of ethanol, acetic acid), irreversible electroporation (IRE) and thermal ablation [radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation] [1–11]. In the latter techniques, one should also include coblation (radiofrequency ionisation used mainly for tumour decompression), irreversible electroporation (IRE) and MR-guided HIFU (high intensity focus ultrasound, which is totally noninvasive). These techniques may act as first-line therapies in certain pathological entities or as attractive adjuncts to conservative therapy, radiotherapy or surgery in other cases.
Prior to performing any ablation, the interventional radiologist should be aware of the tumour histology (benign or malignant), the patient’s general condition and the degree of bone destruction (will consolidation be needed?) [2]. The final decision for malignant cases is made by multidisciplinary oncologic boards. The aim of the ablation (curative versus palliative) should be precisely defined from the beginning of the strategy planning. According to the standards of practice for bone ablation, indications for curative treatments include benign (osteoid osteoma, osteoblastoma <3 cm in diameter, chondroblastoma) and malignant (slow-growing cancers with <3 lesions of <3 cm in diameter each) lesions [2]. Indications for palliative therapies include pain reduction, tumour debulking, decompression and reduction and/or prevention of impeding pathological fractures (where stabilisation will be necessary in combination with the ablation technique) [1–11].
This article will describe the mechanism of action of different ablative technologies applied to the musculoskeletal system, summarise the data concerning the safety and effectiveness of percutaneous ablation techniques for benign and malignant (primary and metastatic) lesions and describe the necessary protective measures.
Ablation techniques
General principles
Ablation in the musculoskeletal system can be quite painful and should be performed under some kind of anaesthesia, ranging from conscious sedation to general anaesthesia. All procedures are performed under extensive local sterility measures and prophylactic antibiotics. Whenever an intact bone cortex is noted the coaxial approach is required; the trocar is either drilled or hammered through the cortex depending on the type of cortical reaction (lytic vs. blastic). Once in position, the needle is removed from the trocar and the ablation instrument is inserted coaxially. It is recommended to pass through the lesion before placing the probe, as the sensitive tip of the ablation probe could be damaged by trying to force it through the lesion. Always remember to move the trocar away from the expected ablation zone in order to avoid having a conductor that will transmit heat from the lesion to the surface, with resultant skin and soft tissues burns. In addition, extreme care should be taken concerning the surrounding structures, especially those sensitive to heat or cold (e.g. nerves). Heating at 45˚C has been shown to be neurotoxic to spinal cord and peripheral nerves [12–14]. Cryoablation can also cause neural damage with temporary neuropraxia occurring at −20˚C and permanent neurological damage at ≤−40˚C [2]. Protective measures include passive thermal protection techniques (thermocouples for temperature monitoring, intraoperative neurological monitoring systems such as neurodiagnostic EEG, EMG and evoked potential electrodes and accessories) or active thermal protection techniques (skin protection, hydrodissection, CO2 or air insulation) [2, 15].
Ethanol ablation
Access is gained to the lesion through a percutaneously placed needle. Ethanol injection causes cellular dehydration, vascular thrombosis and ischaemia; however, the technique is governed by the disadvantage of unpredictable diffusion [8].
Laser ablation
Lasers [neodymium yttrium aluminum garnet (Nd:YAG) diode laser 800–1,000 nm] are coaxially inserted in the tumour and transmit infra-red light energy, which results in protein denaturation and coagulation necrosis [2, 8]. Concerning active protective techniques, gas dissection can be performed with air or CO2 (CO2 is more soluble and a better insulator than air; it is therefore more commonly preferred), hydrodissection is performed with dextrose 5 % (acts as an insulator as opposed to normal saline, which acts as a conductor). All kinds of skin cooling, thermal and neural monitoring can be performed.
Radiofrequency ablation
Straight or expandable percutaneously placed electrodes deliver a high-frequency alternating current, which causes ionic agitation with resultant frictional heat (temperatures of 60–100 ˚C) that produces protein denaturation and coagulation necrosis [8]. Concerning active protective techniques, all kinds of gas dissection can be performed. Hydrodissection is performed with dextrose 5 % (acts as an insulator as opposed to normal saline, which acts as a conductor). All kinds of skin cooling, thermal and neural monitoring can be performed.
Microwave ablation
Straight percutaneously placed antennae deliver electromagnetic microwaves (915 or 2,450 MHz) with resultant frictional heat (temperatures of 60–100 ˚C) that produces protein denaturation and coagulation necrosis [8]. Concerning active protective techniques, all kinds of gas dissection can be performed, whilst hydrodissection is usually avoided (MWA is based on agitation of water molecules for energy transmission). All kinds of skin cooling, thermal and neural monitoring can be performed.
Cryoablation
Straight percutaneously placed cryoprobes deliver room temperature argon gas (for cooling) and helium gas (for thawing) with two cycles of 10-min freezing separated by a 5-min cycle of active thawing usually constituting a typical ablation session [8]. An advantage of cryoablation is that the ice ball is visible under imaging guidance. Also, the technique is governed by significantly lower peri- and post-procedural pain [5]. Disadvantages include the increased cost and time duration [6]. Concerning active protective measures, all kinds of gas dissection can be performed; however, hydrodissection is contraindicated since fluid will freeze when in contact with the ice ball. All kinds of skin warming, thermal and neural monitoring can be performed.
MR-guided HIFU
Focussed ultrasound energy is delivered within the lesion under MR guidace with resultant focal elevated temperatures [8]. An advantage of the technique is the real-time thermal monitoring provided by the MR guidance. MR-guided HIFU sessions are performed with a focussed ultrasound phased-array system for treatment, which is integrated with the MR system. Patients are placed in the optimal position to align the lesion to be treated with the ultrasound transducer located in the MR table. The transducer is housed in an oil bath located in the MR table; a moistened gel pad is used to couple the transducer to the patient’s skin and to eliminate air across the path of the ultrasound beam. Treatment planning is three-dimensional, including a combination of coronal, sagittal and axial sequences with and without fat suppression. In addition, treatment planning takes account of the anticipated energy delivered through the skin to the target lesion. Low subtherapeutic sonications can be used to verify the correct target area and then treatment starts at full therapeutic power.
Irreversible Electroporation (IRE)
Each cell membrane point has a local transmembrane voltage that determines a dynamic phenomenon called electroporation (reversible or irreversible) [16]. Electroporation is manifested by specific transmembrane voltage thresholds related to a given pulse duration and shape. Thus, a threshold for an electronic field magnitude is defined and only cells with higher electric field magnitudes than this threshold are electroporated. IRE produces persistent nano-sized membrane pores compromising the viability of cells [16]. On the other hand, collagen and other supporting structures remain unaffected. The IRE generator produces direct current (25–45 A) electric pulses of high voltage (1,500–3,000 V).
Benign tumours
Osteoid osteoma is a relatively common benign tumour (2-3 % of all bone tumours, 10 % of benign bone tumours) usually seen in children and young adults [17]. Although these small tumours (usually of <1.5 cm diameter) have no or a minimum growth rate, they seem larger in imaging studies because of the surrounding oedema and tissue reaction [17]. Due to the extreme appearance of the surrounding tissues, MRI usually leads to overestimation and occasionally is misleading for the diagnosis; CT seems to be the method of choice for the diagnosis of osteoid osteoma [18]. This benign tumour is quite painful and patients complain of localised pain that is worse in the night and characteristically relieved by NSAIDs (nonsteroid antiinflammatory drugs) [6]. In the literature there are few studies with limited patient numbers reporting disappearance of the pain after conservative therapy even if the imaging findings stay the same with no changes [19–21]. Before deciding to try to treat an osteoid osteoma conservatively, the physician should weigh the potential complications, including not only those from long-term use of NSAIDs but also the chance of muscular atrophy and bone deformity in patients aged <5 years [6, 22].
Radiofrequency ablation was first performed in osteoid osteoma [23, 24]. Ever since, for the therapy of this benign tumour, thermal ablation constitutes a first-line therapy. Concerning osteoid osteoma ablation, throughout the literature there are numerous studies, some with smaller and others with higher numbers of patients, but all of them have quite high pain reduction rates (up to 96 %) and low recurrence rates (∼7 % at 2 years) in common [6, 25–31]. Described thermal ablation techniques for the treatment of osteoid osteoma include the use of monopolar or bipolar RF electrodes, plasma-mediated RF electrodes and laser [32–36]. Recent studies on the use of microwaves and MR-guided HIFU report similar success rates and minimal complications [37–39].
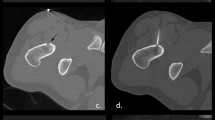
Percutaneous ablation of osteoid osteoma is performed under CT guidance, extended local sterility measures and antibiotic prophylaxis. Access to the nidus is achieved with a trocar that is either hammered or drilled through the intact bone. Once inside the nidus, a bone biopsy needle can be inserted coaxially and a sample obtained to verify the osteoma diagnosis. Then the electrode is inserted coaxially through the trocar, and ablation is performed with a specific protocol resulting in an ablation zone of ∼1 cm diameter (Fig. 1). Potential complications are rare and include iatrogenic damage to the surrounding nerve root or tissues due to the electrode placement, heat effect and size of the bone necrosis [6]. Follow-up of successful ablation is performed clinically and there is no need for imaging follow-up in asymptomatic patients [6].
Osteoid osteoma in the tibia: a CT axial scan: metallic markers were placed on the patient’s skin. b CT axial scan: Once the trocar reaches the lesion, the needle is removed and a bone biopsy needle is inserted coaxially for sampling to verify the diagnosis. c CT axial scan: Then, the radiofrequency electrode is inserted into the lesion coaxially and an ablation protocol specific for osteoid osteoma is performed. d CT axial scan: Follow-up post trocar removal. Notice the trocar tract, which ends up in the lesion
Traditional surgical techniques for osteoid osteoma treatment include wide excision removing a bone block, marginal resection of the entire nidus, curettage or high-speed burr techniques [40]. Comparison of these techniques to percutaneous, minimally invasive, imaging-guided ablation favours the latter in terms of minimum trauma, minimum functional restriction and significantly lower cost [40].
Thermal ablation can be used as a treatment in a variety of benign tumours including osteoblastoma (<3 cm in diameter) (Fig. 2) and chondroblastoma, whilst throughout the literature there are reports of ablation in cases of chondromyxoid fibroma, intracortical chondroma, aneurysmal bone cyst, eosinophilic granuloma and cystic hydroma [2, 6, 41–43]. State-of-the-art reviews report that “essentially any small well defined lesion at imaging can be treated with RF ablation” [6].
Osteoblastoma in the L2-L3 facet joint: a CT axial scan: A 22G spinal needle is inserted inside the epidural space and air is injected through the antimicrobial filter. b Axial CT scan: A trocar is drilled till the lesion. c Axial CT scan: Coaxially, a bipolar radiofrequency electrode is introduced inside the lesion and the ablation protocol is performed. d During the ablation protocol, evoked potentials are used for nerve monitoring
Recent studies upon cryoablation report promising preliminary results whenever the technique is applied as the treatment in extrabdominal desmoids tumours [44, 45]. This kind of treatment for soft tissue tumours seems to be governed by high efficacy concerning local tumour control and pain reduction and at the same time by the reduced complication rate and convalescence rate post-therapeutically [44, 45].
Malignant tumours
The most common malignant bone tumours are metastatic lesions; any malignant neoplasm possesses the capacity to metastasise in the musculoskeletal system [46]. In the general population carcinomas of the breast, prostate, lung and kidney constitute, in decreasing order, ∼75 % of skeletal metastasis cases whilst carcinomas of the prostate, lung and bladder are more common in males and carcinomas of the breast and uterus more common in females [46]. Osseous metastatic disease especially when lytic lesions are involved can result in significant pain and mobility impairment. In most cases the therapeutic goal is palliation with resultant pain reduction and mobility improvement. Alternative therapeutic goals in palliative treatments include tumour reduction/decompression and bone consolidation. Potential therapies include surgery, embolisation, chemotherapy, osteoplasty, ablation, radiotherapy and palliative analgesics. External beam radiotherapy seems to provide at least partial pain relief in 50 % and 80 % of patients with only 30 % reporting complete pain relief [47]. In addition, radiotherapy may result in osteonecrosis or neural damage [48].
Apart from completely eliminating small oligometastatic disease (<3 lesions each measuring <3 cm in diameter), ablation is mainly used for achieving pain reduction to necrotise the interface between the tumour and the pain-sensitive periosteum [2, 3, 5, 6]. Alternative pathophysiologic explanations for pain reduction post ablation include decompression of the tumour volume, a decrease in nerve-stimulating cytokines released by the tumour and inhibition of osteoclast activity [8, 49]. Proper patient selection cannot be emphasised enough; since ablation is a local therapy only patients with localised, at least moderate (>4/10 numeric visual scale units) pain should be treated. Absolute contraindications include coagulopathy disorders, skin infection, immunosuppression and the absence of a safe path to the lesion [50].
Percutaneous ablation of malignant metastatic lesions is performed under imaging guidance, extended local sterility measures and antibiotic prophylaxis. Whenever the ablation zone is expected to extend up to 1 cm close to critical structures (e.g. the nerve root, skin, etc.), all the necessary thermal protection techniques should be applied (Fig. 3).
a Painful soft tissue mass infiltrating the left T10 posterior rib. b A microwave antenna is percutaneously inserted inside the mass. Due to the proximity to the skin a sterile glove filled with cold water is placed over the skin. c CT axial scan 3 months after the ablation session: Notice the significant size reduction of the mass along with new bone formation around the left T10 posterior rib (similar to heterotopic ossification)
Recent reviews and studies on ablation report promising results whenever the technique is applied as treatment in secondary lesions located in the soft tissues resulting in local tumour control and pain reduction with reduced complication rates and convalescence rates post-therapeutically [8, 45, 50–53]. Similarly, studies in the literature on percutaneous ablation (RFA, MWA, MR-guided HIFU and cryoablation) of metastatic painful bony lesions report significant pain reduction with minimal complications (Fig. 4) [11, 51–53]. To date, there seems to be no difference in the efficacy of one technique over the other [9].
A recent application of ablation techniques, more specifically of HIFU, is its use as a limb-salvaging treatment in patients with osteosarcoma with resultant changes in symptoms and survival time [54]. However, further and more extended studies are necessary for confirmation.
The cost of tumour necrosis in bone lesions treated with ablation techniques is bone weakening. In order to avoid post-therapeutic osteonecrosis with pathologic fractures, cement augmentation should be combined with ablation especially in the spine and other weight-bearing areas [2, 3, 5, 6, 8, 9, 50, 55]. Furthermore, in certain locations, due to the direction of the forces applied upon weight bearing, cement augmentation might be insufficient and should be combined with further augmentation with metallic instrumentation; preliminary studies upon such combinations report very promising results [56–58]. Whenever combining ablation with cementation at the same session, it is necessary to allow enough time for the tumour temperature to return back to normal in order to avoid untimely cement polymerisation [50].
In order to enhance local tumour control, ablation can be combined with transarterial chemoembolisation as well (especially for hypervascular lesions usually originating from thyroid, renal cell or hepatocellular carcinomas) [59, 60]. Whenever combining ablation with TACE or any other embolisation technique, the intravascular technique usually precedes the ablation with/without cement augmentation [50].
Protective techniques
Protective measures include various techniques of insulation and temperature or nerve function monitoring aiming at protecting vulnerable structures. Passive thermal protection techniques include continuous monitoring of the temperature in the area of interest by thermocouples or of nervous function by monitoring systems such as neurodiagnostic EEG, EMG and evoked potential electrodes with accessories [61, 62]. Measurement of the temperature in proximity to a neural structure or of the nerve’s functional ability during the ablation provides valuable information for a safe and efficient session. Heating over 45 °C or cooling below 10 °C can be neurotoxic to the spinal cord and the peripheral nerves [2].
Active thermal protection techniques include gas dissection, hydrodissection and warming/cooling modes for skin protection (subcutaneous fluid injection, application of a sterile glove with warm or cold saline).
During gas dissection, CO2 or air is injected by means of a 22G spinal needle for dissecting vulnerable structures away from the ablation zone. CO2 is a better insulator than air, which is less soluble and might result in emboli formation [61, 62]. For both gases use of an antimicrobial filter is optional. Dissection by gas is not governed by heating or cooling properties but it is only used in order to increase the distance between the ablation zone and a certain structure [61, 62]. In addition, during the ablation session the injected gas might be absorbed and therefore additional injection might be needed. Gas dissection is the active insulation of choice whenever cryoablation is performed and in most cases of microwave ablation. Gas dissection increases the distance between the expected ablation zone and close nerve structures; in addition, it is more commonly preferred over fluid dissection whenever a spinal or paraspinal lesion is to be ablated and the ablation zone is expected to extend near the epidural space.
Fluid dissection creates the distance necessary for a safe ablation zone, provides cooling or warming (depending on the fluid’s temperature) as well as an occasional insulating effect. In bone and soft tissue ablation this technique can be used in epidural (nerve protection) or articular (cartilage protection) spaces. In addition, it can increase the distance between the expected ablation zone and a close nerve structure. For increased visualisation a small amount of contrast medium can be diluted in the fluid [61, 62]. Whenever fluid dissection is required in ablation performed by radiofrequency energy, dextrose in water (D/W 5 %) is preferred over saline solution because of the latter’s high electrical conductivity [61, 62]. For microwave ablation, any fluid can be used as long as it is distributed at least 2 cm away from the active tip of the antenna. Fluid dissection cannot be used in cryoablation because the used fluid freezes when it comes in contact with the ice ball.
If ablating superficial tumours the skin should be protected in order to avoid and prevent painful burns or frostbite. Skin seems to tolerate low better than high temperatures [61, 62]. Techniques for preventing skin lesions during ablation include positioning sterile gloves containing warm or cooled fluid over the expected ablation zone (for cryoablation and RFA/MWA respectively). In addition, subdermal injection of local anaesthetic or D/W 5 % increases the distance between the ablation zone and skin.
Conclusion
Percutaneous ablation techniques constitute a safe and efficacious minimally invasive therapy for the treatment of osteoid osteoma and an attractive adjunct in the therapeutic armamentarium of small benign lesions or of oligometastatic bone disease. In addition, thermal ablation can act as palliative therapy in the rest of the cases. Primary or metastatic malignant bone lesions (especially osteolytic) can become very painful. The therapeutic armamentarium includes conservative therapy (analgesics), surgery, chemotherapy, radiotherapy, embolisation, ablation and cementation. External beam radiotherapy, although used frequently, provides moderate pain relief. Tumour necrosis by means of percutaneous ablation techniques seems an effective, safe and feasible technique for the treatment of painful bony lesions. Ablation (RFA, MWA, cryoablation) can treat such lesions and provide local tumour control, performed either alone or in combination with surgical resection or other percutaneous techniques. Specifically for the spine and other weight-bearing locations, ablation should be combined with cement augmentation and occasionally also with further augmentation with metallic instrumentation (which can also be percutaneous). Protection of surrounding sensitive or critical structures is necessary whenever the ablation zone is expected to extend close to these structures. Thorough knowledge of each ablation technique and of all available protective techniques (both passive and active, and in addition how they can be combined) is mandatory for a safe and successful procedure.
References
Papagelopoulos PJ, Mavrogenis AF, Galanis EC, Kelekis NL, Wenger DE, Sim FH, Soucacos PN (2005) Minimally invasive techniques in orthopedic oncology: radiofrequency and laser thermal ablation. Orthopedics 28(6):563–568
Gangi A, Tsoumakidou G, Buy X, Quoix E (2010) Quality improvement guidelines for bone tumour management. Cardiovasc Intervent Radiol 33(4):706–713
Kelekis AD, Somon T, Yilmaz H, Bize P, Brountzos EN, Lovblad K, Ruefenacht D, Martin JB (2005) Interventional spine procedures. Eur J Radiol 55(3):362–383
Carrafiello G, Laganà D, Pellegrino C, Fontana F, Mangini M, Nicotera P, Petullà M, Bracchi E, Genovese E, Cuffari S, Fugazzola C (2009) Percutaneous imaging-guided ablation therapies in the treatment of symptomatic bone metastases: preliminary experience. Radiol Med 114(4):608–625
Gangi A, Buy X (2010) Percutaneous bone tumor management. Semin Intervent Radiol 27(2):124–136
Rosenthal D, Callstrom MR (2012) Critical review and state of the art in interventional oncology: benign and metastatic disease involving bone. Radiology 262(3):765–780
Carrafiello G, Laganà D, Ianniello A, Nicotera P, Fontana F, Dizonno M, Cuffari S, Fugazzola C (2009) Radiofrequency thermal ablation for pain control in patients with single painful bone metastasis from hepatocellular carcinoma. Eur J Radiol 71(2):363–368
Kurup AN, Callstrom MR (2010) Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol 27(3):276–284
Masala S, Guglielmi G, Petrella MC, Mastrangeli R, Meschini A, Anselmetti GC, Bartolucci DA, Mammucari M, Manenti G, Simonetti G (2011) Percutaneous ablative treatment of metastatic bone tumours: visual analogue scale scores in a short-term series. Singapore Med J 52(3):182–189
Kurup AN, Callstrom MR (2010) Ablation of skeletal metastases: current status. J Vasc Interv Radiol 21(8 Suppl):S242–250
Napoli A, Anzidei M, Marincola BC, Brachetti G, Noce V, Boni F, Bertaccini L, Passariello R, Catalano C (2013) MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics 33(6):1555–1568
Dupuy DE, Hong R, Oliver B et al (2000) Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR 175:1263–1266
Diehn FE, Neeman Z, Hvizda JL et al (2003) Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol 14:1569–1576
Nakatsuka A, Yamakado K, Takaki H, Uraki J, Makita M, Oshima F, Takeda K (2009) Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: a prospective study. Cardiovasc Intervent Radiol 32(1):70–75
Tsoumakidou G, Garnon J, Ramamurthy N, Buy X, Gangi A (2013) Interest of electrostimulation of peripheral motor nerves during percutaneous thermal ablation. Cardiovasc Intervent Radiol 36(6):1624–1628
Thompson KR, Cheung W, Ellis SJ et al (2011) Investigation of the safety of irreversible electroporation in humans. JVIR 22:611–621
Gamet I (2006) The management of osteoid osteoma: updates and controversies. Curr Opin Pediatr 18:36–41
Lee MH, Ahn JM, Chung HW et al (2007) Osteoid osteoma treated with percutaneous radiofrequency ablation: MR imaging follow-up. Eur J Radiol 64(2):309–314
Kneisl JS, Simon MA (1992) Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am 74(2):179–185
Jayakumar P, Harish S, Nnadi C, Noordeen H, Saifuddin A (2007) Symptomatic resolution of spinal osteoid osteoma with conservative management: imaging correlation. Skeletal Radiol 36(Suppl 1):S72–S76
Goto T, Shinoda Y, Okuma T et al (2011) Administration of nonsteroidal anti-inflammatory drugs accelerates spontaneous healing of osteoid osteoma. Arch Orthop Trauma Surg 131(5):619–625
Norman A, Dorfman HD (1975) Osteoid-osteoma inducing pronounced overgrowth and deformity of bone. Clin Orthop Relat Res 110:233–238
Rosenthal DI, Alexander A, Rosenberg AE, Springfield D (1992) Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology 183(1):29–33
Rosenthal DI, Springfield DS, Gebhardt MC, Rosenberg AE, Mankin HJ (1995) Osteoid osteoma: percutaneous radio-frequency ablation. Radiology 197(2):451–454
Barei DP, Moreau G, Scarborough MT, Neel MD (2000) Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop Relat Res 373:115–124
Kjar RA, Powell GJ, Schilcht SM, Smith PJ, Slavin J, Choong PF (2006) Percutaneous radiofrequency ablation for osteoid osteoma: experience with a new treatment. Med J Aust 184(11):563–565
Rimondi E, Bianchi G, Malaguti MC et al (2005) Radiofrequency thermoablation of primary non-spinal osteoid osteoma: optimization of the procedure. Eur Radiol 15(7):1393–1399
Mahnken AH, Bruners P, Delbrück H, Günther RW (2011) Radiofrequency ablation of osteoid osteoma: initial experience with a new monopolar ablation device. Cardiovasc Intervent Radiol 34(3):579–584
Mylona S, Patsoura S, Galani P, Karapostolakis G, Pomoni A, Thanos L (2010) Osteoid osteomas in common and in technically challenging locations treated with computed tomography-guided percutaneous radiofrequency ablation. Skeletal Radiol 39(5):443–449
Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, Menendez L (2009) Thermal ablation of osteoid osteoma: overview and step-by-step guide. Radiographics 29(7):2127–2141
Donkol RH, Al-Nammi A, Moghazi K (2008) Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr Radiol 38(2):180–185
Witt JD, Hall-Craggs MA, Ripley P et al (2000) Interstitial laser photocoagulation for the treatment of osteoid osteoma. J Bone Joint Surg (Br) 82B:1125–1128
Gangi A, Alizadeh H, Wong L, Buy X, Dietemann JL, Roy C (2007) Osteoid osteoma: percutaneous laser ablation and follow-up in 114 patients. Radiology 242(1):293–301
Maurer MH, Gebauer B, Wieners G, De Bucourt M, Renz DM, Hamm B, Streitparth F (2012) Treatment of osteoid osteoma using CT-guided radiofrequency ablation versus MR-guided laser ablation: a cost comparison. Eur J Radiol 81(11):e1002–e1006
Mahnken AH, Tacke JA, Wildberger JE, Günther RW (2006) Radiofrequency ablation of osteoid osteoma: initial results with a bipolar ablation device. J Vasc Interv Radiol 17(9):1465–1470
Dasenbrock HH, Gandhi D, Kathuria S (2012) Percutaneous plasma mediated radiofrequency ablation of spinal osteoid osteomas. J Neurointerv Surg 4(3):226–228.
Kostrzewa M, Diezler P, Michaely H, Rathmann N, Attenberger UI, Schoenberg SO, Diehl SJ (2013) Microwave ablation of osteoid osteomas using dynamic MR imaging for early treatment assessment: preliminary experience. J Vasc Interv Radiol 25(1):106–111
Basile A, Failla G, Reforgiato A, Scavone G, Mundo E, Messina M, Caltabiano G, Arena F, Ricceri V, Scavone A, Masala S (2013) The use of microwaves ablation in the treatment of epiphyseal osteoid osteomas. Cardiovasc Intervent Radiol 30, PMID:23989501
Napoli A, Mastantuono M, Cavallo Marincola B, Anzidei M, Zaccagna F, Moreschini O, Passariello R, Catalano C (2013) Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 267(2):514–521
Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF (1997) CT-controlled thermocoagulation of osteoid osteoma in comparison with traditional methods. Z Orthop Ihre Grenzgeb 135(6):522–527
Ramnath RR, Rosenthal DI, Cates J, Gebhardt M, Quinn RH (2002) Intracortical chondroma simulating osteoid osteoma treated by radiofrequency. Skeletal Radiol 31(10):597–602
Corby RR, Stacy GS, Peabody TD, Dixon LB (2008) Radiofrequency ablation of solitary eosinophilic granuloma of bone. AJR Am J Roentgenol 190(6):1492–1494
Cable BB, Mair EA (2001) Radiofrequency ablation of lymphangiomatous macroglossia. Laryngoscope 111(10):1859–1861
Cornelis F, Italiano A, Al-Ammari S, Kind M, Stoeckle E, Gangi A, Palussière J, Bui BN (2012) Successful iterative percutaneous cryoablation of multiple extraabdominal desmoid tumors in a patient with Gardner syndrome. J Vasc Interv Radio l23(8):1101–1103.
Cornelis F, Havez M, Lippa N, Al-Ammari S, Verdier D, Carteret T, Amoretti N, Gangi A, Palussiere J, Hauger O, Grenier N (2013) Radiologically guided percutaneous cryotherapy for soft tissue tumours: a promising treatment. Diagn Interv Imaging 94(4):364–370.
Radford M, Gibbons CL (2002) Management of skeletal metastases. Hosp Med 63(12):722–725
S. Lutz, E. Chowb (2012) A review of recently published radiotherapy treatment guidelines for bone metastases: contrasts or convergence? J Bone Oncol 1:18–23
Frassica DA (2003) General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res (415 Suppl):S158–S164.
Callstrom MR, Charboneau JW, Goetz MP, et al. (2002) Painful metastases involving bone: feasibility of percutaneous CT and US-guided radio-frequency ablation. Radiology 224(1):87–97
Kelekis AD, Martin JB, Filippiadis DF (2013) Ablation and combination treatments of bony lesions. In: Matthew AM, Kieran PJM, Kenneth RT (eds) Image guided interventions: expert radiology series, Elsevier, pp 1182–1187
Kastler A, Alnassan H, Pereira PL, Alemann G, Barbé DA, Aubry S, Tiberghien F, Kastler B (2013) Analgesic effects of microwave ablation of bone and soft tissue tumors under local anesthesia. Pain Med 14(12):1873–1881
Pusceddu C, Sotgia B, Fele RM, Melis L (2013) Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol 24(2):229–233
Nicholas Kurup A, Callstrom MR (2013) Ablation of musculoskeletal metastases: pain palliation, fracture risk reduction, and oligometastatic disease. Tech Vasc Interv Radiol 16(4):253–261
Li C, Wu P, Zhang L, Fan W, Huang J, Zhang F (2009) Osteosarcoma: limb salvaging treatment by ultrasonographically guided high intensity focused ultrasound. Cancer Biol Ther 12:1102–1108
Hoffmann RT, Jakobs TF, Trumm C, Weber C, Helmberger TK, Reiser MF (2008) Radiofrequency ablation in combination with osteoplasty in the treatment of painful metastatic bone disease. J Vasc Interv Radiol 19(3):419–425
Abdel-Aal AK, Underwood ES, Saddekni S (2012) Use of cryoablation and osteoplasty reinforced with Kirschner wires in the treatment of femoral metastasis. Cardiovasc Intervent Radiol 35(5):1211–1215
Deschamps F, Farouil G, Hakime A, Teriitehau C, Barah A, de Baere T (2012) Percutaneous stabilization of impending pathological fracture of the proximal femur. Cardiovasc Intervent Radiol 35(6):1428–1432
Anselmetti GC, Manca A, Chiara G, Tutton S, Iussich G, Gino G, Grignani G, Ortega C, Moselli N, Regge D (2011) Painful pathologic fracture of the humerus: percutaneous osteoplasty with bone marrow nails under hybrid computed tomography and fluoroscopic guidance. J Vasc Interv Radiol 22(7):1031–1034
Alda T, Kamran A (2007) Palliative interventions for pain in cancer patients. Semin Intervent Radiol 24(4):419–429
Lee JH, Stein M, Roychowdhury S (2013) Percutaneous treatment of a sacral metastasis with combined embolization, cryoablation, alcohol ablation and sacroplasty for local tumor and pain control. Interv Neuroradiol 2:250–253
Tsoumakidou G, Buy X, Garnon J, Gangi A (2011) Tumor thermal ablation: Insulation and temperature monitoring. Scientific Exhibit ESR 2011. doi:10.1594/ecr2011/C-2281
Filippiadis DK, Mazioti A, Velonakis G, Papakonstantinou O, Malagari A, Kelekis NL, Kelekis AD (2013) Percutaneous image-guided ablation of bone and soft tissue tumors: how to avoid complications. Scientific Exhibit ESSR 2013. doi:10.1594/essr2013/P-0127
Author information
Authors and Affiliations
Corresponding author
Additional information
Reference to the electronic poster
P-0127 Percutaneous image-guided ablation of bone and soft tissue tumors: how to avoid complications DOI: 10.1594/essr2013/P-O127
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Filippiadis, D.K., Tutton, S., Mazioti, A. et al. Percutaneous image-guided ablation of bone and soft tissue tumours: a review of available techniques and protective measures. Insights Imaging 5, 339–346 (2014). https://doi.org/10.1007/s13244-014-0332-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13244-014-0332-6